The complex of rice immune receptor RGA5-HMA8 with rice blast effector protein AVR-PikD
Zhang, X., Liu, J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Disease resistance protein RGA5 | 75 | Oryza sativa | Mutation(s): 0 Gene Names: RGA5 | 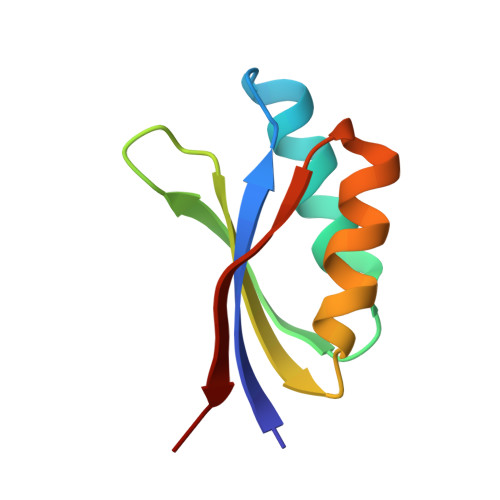 | |
UniProt | |||||
Find proteins for F7J0N2 (Oryza sativa subsp. japonica) Explore F7J0N2 Go to UniProtKB: F7J0N2 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | F7J0N2 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| AVR-Pik protein | B [auth C] | 81 | Pyricularia oryzae | Mutation(s): 0 Gene Names: AVR-Pik, AvrPik, Pikm, Pikp | 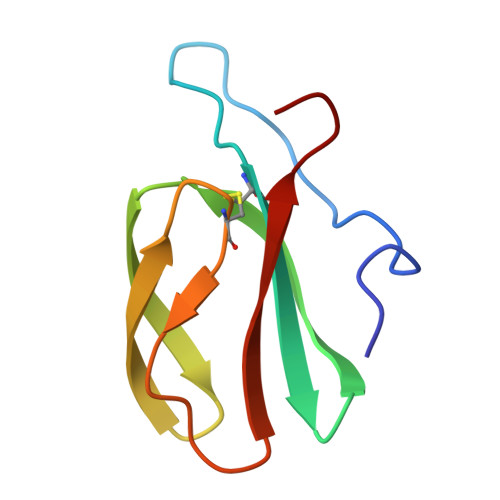 |
UniProt | |||||
Find proteins for A0AA97NPM3 (Pyricularia oryzae (strain Y34)) Explore A0AA97NPM3 Go to UniProtKB: A0AA97NPM3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0AA97NPM3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 66.41 | α = 90 |
| b = 66.41 | β = 90 |
| c = 84.446 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data scaling |
| HKL-2000 | data reduction |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | -- |