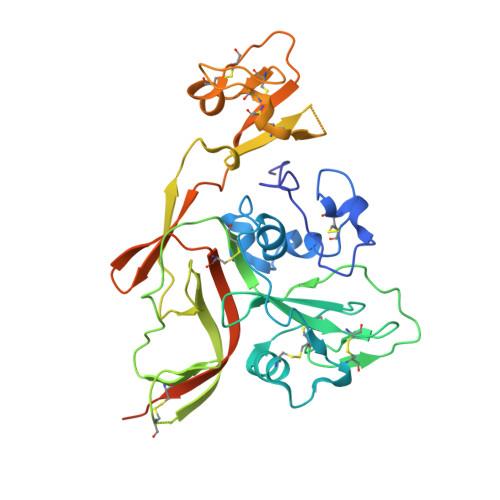
Structural basis for recognition of Rift Valley fever virus determine neutralization potency Gn protein by a human neutralizing monoclonal antibody encoded by a kappa light chain
Paesen, G.C., Chapman, N.S., Westover, J.L.B., McMillan, C.M., Kuzmina, N.A., Myers, L., Stass, R., Montgomery, J.M., Bukreyev, A., Hartman, A.L., Gowen, B.B., Crowe, J.E., Bowden, T.A.To be published.