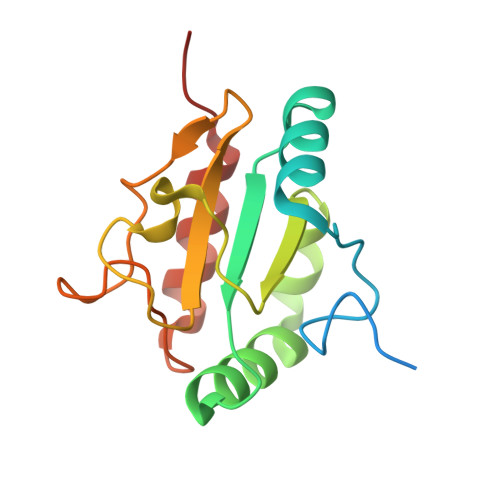
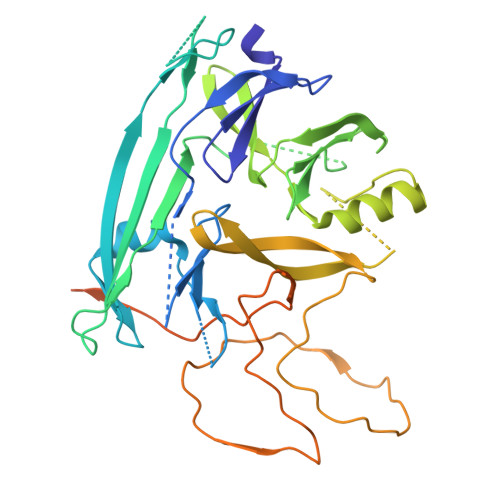
A structural basis for chaperone repression of stress signaling from the endoplasmic reticulum.
Neidhardt, L., Tung, J., Kuchersky, M., Milczarek, J., Kargas, V., Stott, K., Rosenzweig, R., Ron, D., Yan, Y.(2025) Mol Cell 85: 4047
- PubMed: 41135511
- DOI: https://doi.org/10.1016/j.molcel.2025.09.032
- Primary Citation of Related Structures:
9I3F, 9I3U - PubMed Abstract:
The endoplasmic reticulum (ER) unfolded protein response (UPR) is tuned by the balance between unfolded proteins and chaperones. Reserve chaperones suppress UPR transducers via their stress-sensing luminal domains, but the underlying mechanisms remain unclear. The ER chaperone AGR2 is known to repress the UPR transducer IRE1β. Here, structural prediction, X-ray crystallography, and NMR spectroscopy identify critical interactions between an AGR2 monomer and a regulatory loop in IRE1β's luminal domain. However, in the repressive complex, it is an AGR2 dimer that binds IRE1β. Cryoelectron microscopy (cryo-EM) reconstruction explains this feature: one AGR2 protomer engages the regulatory loop, while the second asymmetrically binds IRE1β's luminal domain's C terminus, blocking IRE1β-activating dimerization. Molecular dynamic simulations indicate that the second, disruptive AGR2 protomer exploits rare fluctuations in the IRE1β dimer that expose its binding site. Thus, AGR2 disrupts IRE1β dimers to suppress the UPR, priming the system for activation by chaperone clients that compete for AGR2.
- Cambridge Institute for Medical Research, University of Cambridge, Cambridge CB2 0XY, UK. Electronic address: lneidhar@ic.ac.uk.
Organizational Affiliation: