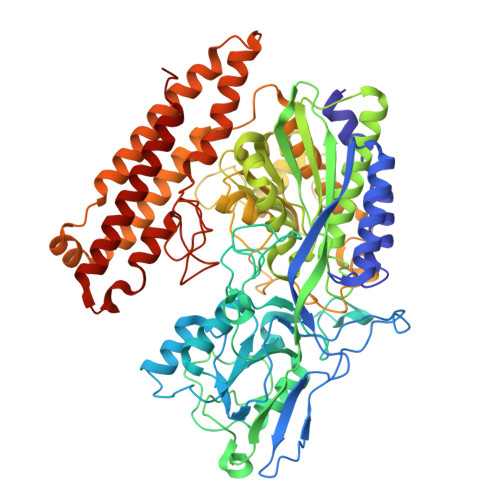
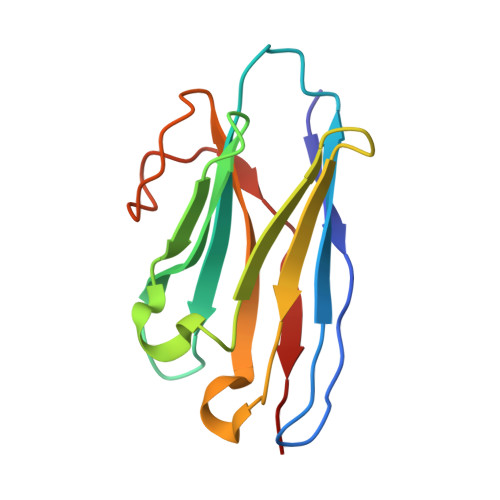
Structural analysis of nanobody interactions with their prostate-specific membrane antigen binding epitopes.
Alon-Zchut, G., Zalk, R., Huynh, T.T., Zalutsky, M.R., Weizmann, Y., Zarivach, R., Papo, N.(2025) Int J Biol Macromol 320: 145693-145693
- PubMed: 40609945
- DOI: https://doi.org/10.1016/j.ijbiomac.2025.145693
- Primary Citation of Related Structures:
9HLW, 9HVI, 9HVK, 9HVL - PubMed Abstract:
Prostate-specific membrane antigen (PSMA), overexpressed in prostate cancer, is a promising target for diagnostics and therapy. However, the monoclonal antibodies in current use for PSMA targeting and inhibition have suboptimal activities due to their poor tissue and cell penetration and slow normal tissue clearance. Potentially superior alternatives are nanobodies (NBs), the single-chain variable domains of heavy-chain antibodies derived from camelids. The advantages of NBs include small size (~15 kDa), ability to bind hidden epitopes, and rapid clearance. In contrast to most known PSMA inhibitors, which bind to the same catalytic site in PMSA, NBs can bind to different PSMA epitopes, facilitating heterovalent binding strategies that could enhance their therapeutic and diagnostic potential. The objective of this study was to map these binding epitopes and hence to acquire an atomic-resolution understanding of NB-PMSA binding by investigating the structural interactions between PSMA and three NBs (NB7, NB8, and NB37). Using cryo-electron microscopy to generate high-resolution structures of NB-PSMA complexes, we found that NB7 had the highest affinity for PSMA due to a larger interface and to stabilizing interactions, including salt bridges and π-π stacking. Notably, we also found that NB7 and NB8 can bind simultaneously to different PSMA epitopes without interfering with the function of PSMA (which is still not completely known), opening the way for the development of theranostic applications for prostate cancer treatment and imaging. Importantly, NB7 binds specifically to human PSMA but not to murine PSMA, due to key amino acid differences responsible for its species specificity.
- Avram and Stella Goldstein-Goren Department of Biotechnology Engineering, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel.
Organizational Affiliation: