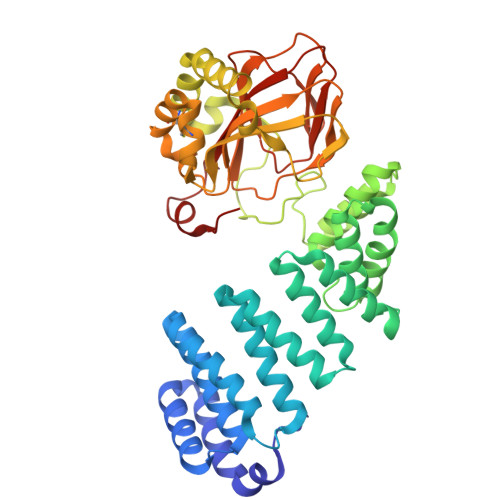
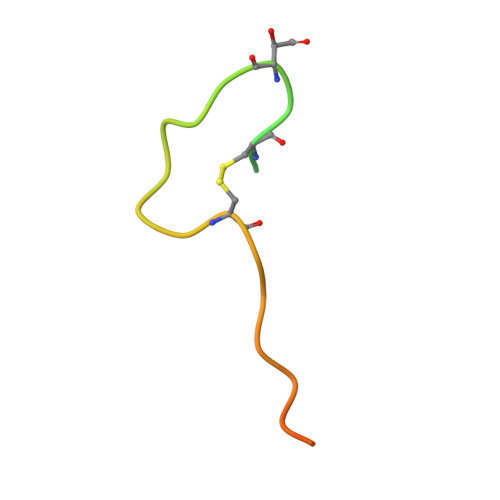
Aspartyl/Asparaginyl beta-hydroxylase (AspH) in complex with Fe, 2-oxoglutarate, succinate and the hydroxylated product of Factor X derived peptide fragment, 12 h O2 exposure
de Munnik, M., Brasnett, A., Rabe, P., Brewitz, L., Park, J., Schofield, C.J., Zhou, T.To be published.