ManDH5 E303Q in complex with mannotetraose after co-crystalliztion with mannotetraose at 1.6 angstroms resolution a beta-D-Mannanase of GH5 family from Dictyoglomus thermophilium
Sivron, Y., Romano, A., Shoham, Y., Shoham, G.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DUF5060 domain-containing protein | 568 | Dictyoglomus thermophilum | Mutation(s): 1 Gene Names: ENW00_02810 | 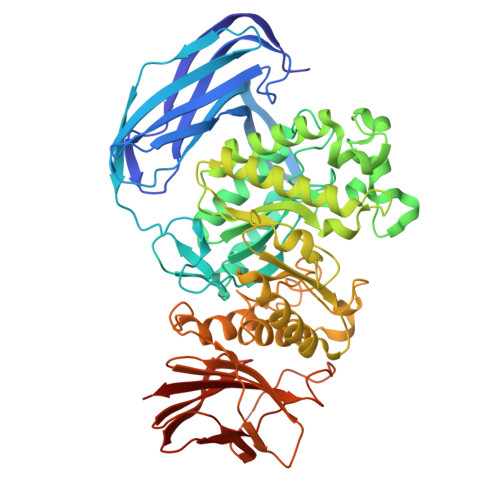 | |
UniProt | |||||
Find proteins for A0A7C3MIF0 (Dictyoglomus thermophilum) Explore A0A7C3MIF0 Go to UniProtKB: A0A7C3MIF0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A7C3MIF0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| BMA (Subject of Investigation/LOI) Query on BMA | C [auth A] | beta-D-mannopyranose C6 H12 O6 WQZGKKKJIJFFOK-RWOPYEJCSA-N |  | ||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_900010 Query on PRD_900010 | B | alpha-maltotetraose | Oligosaccharide / Substrate analog |  | |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 94.946 | α = 90 |
| b = 99.337 | β = 90 |
| c = 153.553 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| xia2 | data scaling |
| PHASER | phasing |
| Coot | model building |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |