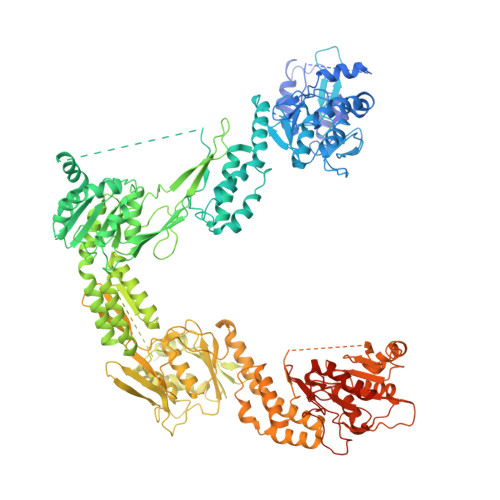
CryoEM structure and small-angle X-ray scattering analyses of porcine retinol-binding protein 3.
Kaushik, V., Gessa, L., Kumar, N., Pinkas, M., Czarnocki-Cieciura, M., Palczewski, K., Novacek, J., Fernandes, H.(2025) Open Biol 15: 240180-240180
- PubMed: 39837501
- DOI: https://doi.org/10.1098/rsob.240180
- Primary Citation of Related Structures:
9FRX - PubMed Abstract:
The vertebrate visual cycle hinges on enzymatically converting all- trans -retinol (at-ROL) into 11- cis -retinal (11c-RAL), the chromophore that binds to opsins in photoreceptors, forming light-responsive pigments. When struck by a photon, these pigments activate the phototransduction pathway and initiate the process of vision. The enzymatic isomerization of at-ROL, crucial for restoring the visual pigments and preparing them to receive new light stimuli, relies on various enzymes found in both the photoreceptors and retinal pigment epithelium cells. To function effectively, retinoids must shuttle between these two cell types. Retinol-binding protein 3 (RBP3), located in the interphotoreceptor matrix, probably plays a pivotal role in this transport mechanism. Comprised of four retinoid-binding modules, RBP3 also binds fatty acids, potentially aiding retinal function by facilitating the loading and unloading of different retinoids at specific cell types thereby directing the cycle. In this study, we present a 3.67 Å cryoEM structure of porcine RBP3, along with molecular docking analysis and corroborative in-solution small-angle X-ray scattering data for titration of RBP3 with relevant ligands, that also give insights on RBP3 conformational adaptability.
- Institute of Physical Chemistry, Polish Academy of Sciences , Warsaw, Poland.
Organizational Affiliation: