Crystal structure of arginine kinases To be published.
Schooltink, L., Todorovic, N., Sagmeister, T., Hofer, G., Keller, W.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| arginine kinase | 357 | Sarcoptes scabiei | Mutation(s): 0 Gene Names: SSS_5952 EC: 2.7.3.3 | 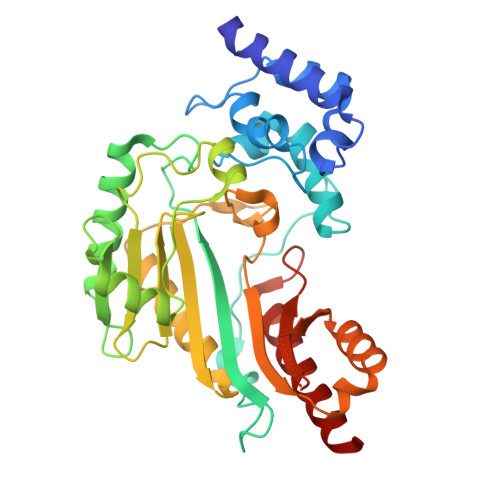 | |
UniProt | |||||
Find proteins for A0A834RHR6 (Sarcoptes scabiei) Explore A0A834RHR6 Go to UniProtKB: A0A834RHR6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A834RHR6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 48.476 | α = 90 |
| b = 80.904 | β = 90 |
| c = 100.504 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Austrian Science Fund | Austria | F4604 |
| Austrian Science Fund | Austria | Doc130 |