Cell attachment and tail contraction of S. aureus phage phi812
Binovsky, J., Siborova, M., Skubnik, K., Novacek, J., Plevka, P.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Major tail sheath protein | 587 | Staphylococcus phage 812 | Mutation(s): 0 | 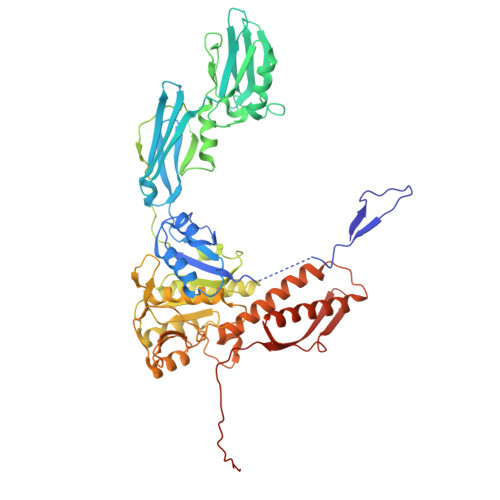 | |
UniProt | |||||
Find proteins for A0A0U1WZ79 (Staphylococcus phage 812) Explore A0A0U1WZ79 Go to UniProtKB: A0A0U1WZ79 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A0U1WZ79 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | |
| RECONSTRUCTION | RELION | 3.1 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| European Research Council (ERC) | European Union | 101043452 |
| Other government | Czech Republic | LX22NP05103 |