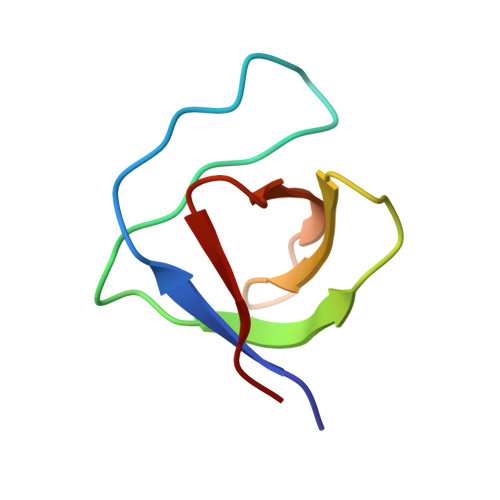
A Triple-pose Complex Between an Extended WIP Motif and a C-terminal SH3 Domain Modulates Cortactin Activity.
Sokolik, C.G., Chill, J.H.(2025) J Mol Biology 437: 168984-168984
- PubMed: 39914658
- DOI: https://doi.org/10.1016/j.jmb.2025.168984
- Primary Citation of Related Structures:
9EZN, 9EZO, 9EZP - PubMed Abstract:
The central domain of WASp-interacting protein (WIP) interacts with the cortactin SH3 domain through a previously undefined binding motif. This interaction affects extracellular matrix (ECM) degradation and the invasive phenotype of cells. Here, using NMR-based methods, we identify the major WIP epitope modulating this binding event as residues 168-183, an unexpectedly long segment uncharacteristic of SH3 peptidic ligands. A scanning mutagenesis analysis showed that peptide binding 'hotspots' are distributed throughout the binding sequence. To uncover the structural basis of WIP-cortactin recognition we utilized edited-filtered NOESY experiments to determine the structure of the intermediate-affinity SH3/peptide complex. Analysis of the NOESY pattern suggests that the peptide sequence dictates three interchanging binding modes, two oppositely oriented canonical poses involving N-terminal interactions, corresponding to class I and class II complexes, and a non-canonical pseudo-class II pose involving C-terminal interactions. The latter pose highlights the importance of the hydrophobic surface adjacent to the canonical binding grooves and accounts for the extended binding motif. Design of mutant peptides with increased affinity based on this multi-conformational complex demonstrates how these structural insights may impact design of improved inhibitors of the WIP-cortactin interaction with potential therapeutic applications.
- Department of Chemistry, Bar Ilan University, Ramat Gan 52900 Israel.
Organizational Affiliation: