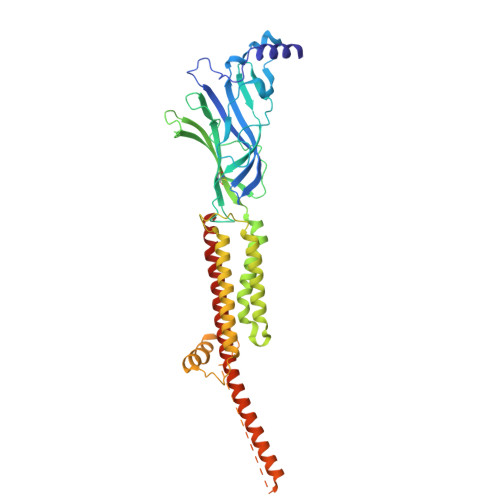
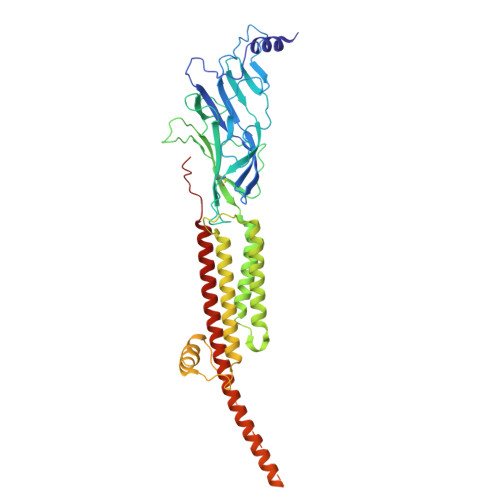
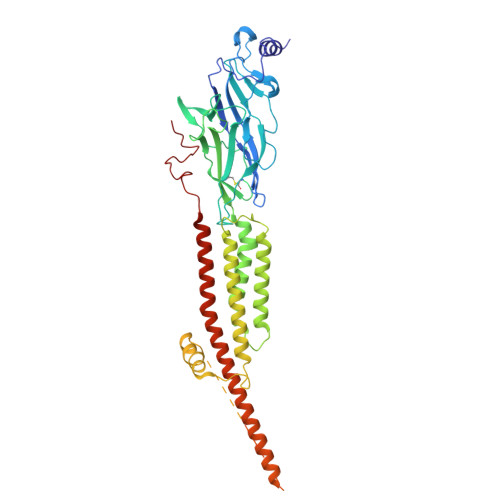
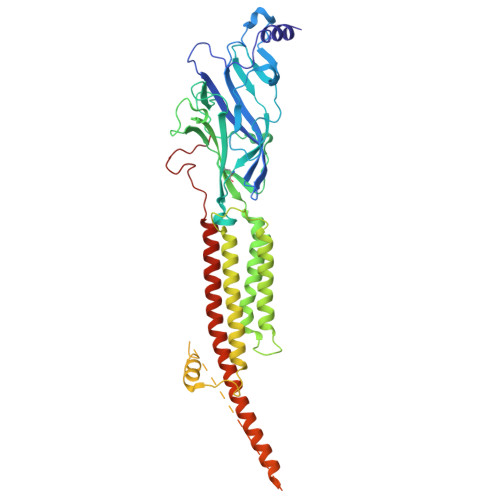
Asynchronous subunit transitions prime acetylcholine receptor activation.
Thompson, M.J., Tessier, C.J.G., Ananchenko, A., Henault, C., Emlaw, J.R., Dehez, F., Zarkadas, E., daCosta, C.J.B., Nury, H., Baenziger, J.E.(2026) Science 391: eadw1264-eadw1264
- PubMed: 41037590
- DOI: https://doi.org/10.1126/science.adw1264
- Primary Citation of Related Structures:
9E3E, 9E3F, 9E3G - PubMed Abstract:
Communication at synapses is facilitated by postsynaptic receptors, which convert a chemical signal into an electrical response. For ligand-gated ion channels, agonist binding triggers rapid transitions through intermediate states leading to a transient open-pore conformation, with these transitions shaping the post-synaptic response. Here, we determine structures of the muscle-type nicotinic acetylcholine receptor in unliganded, mono-liganded, and di-liganded states. Agonist binding to a single site stabilizes a closed structure where an entire principal agonist-binding subunit transitions to an active-like conformation, while the other unoccupied principal subunit remains inactive, albeit poised for activation. Uniting this intermediate structure with single-channel recordings informs a sequential activation mechanism where asynchronous subunit transitions prime the receptor for activation, a finding with implications for an entire superfamily of pentameric ligand-gated ion channels.
- Department of Biochemistry, Microbiology, and Immunology, University of Ottawa, Ottawa, ON, Canada.
Organizational Affiliation: