Structure of the twin-arginine protein translocation pathway core complex and the molecular basis for substrate recognition
Deme, J.C., Bryant, O.J., Berks, B.C., Lea, S.M.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Sec-independent protein translocase protein TatB | A [auth B], F [auth D], G [auth F] | 171 | Escherichia coli | Mutation(s): 0 Gene Names: tatB | 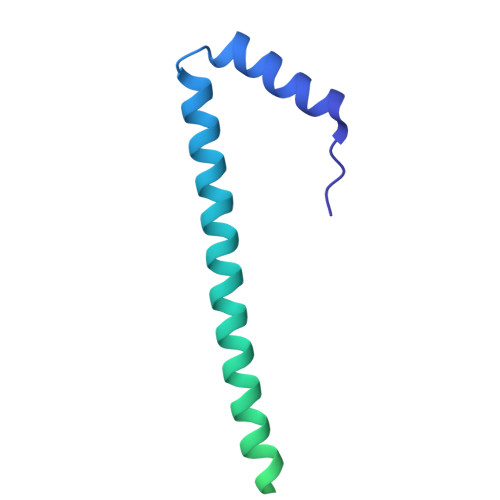 |
UniProt | |||||
Find proteins for P69425 (Escherichia coli (strain K12)) Explore P69425 Go to UniProtKB: P69425 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P69425 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Sec-independent protein translocase protein TatC | B [auth A], D [auth C], E | 266 | Escherichia coli | Mutation(s): 0 Gene Names: tatC | 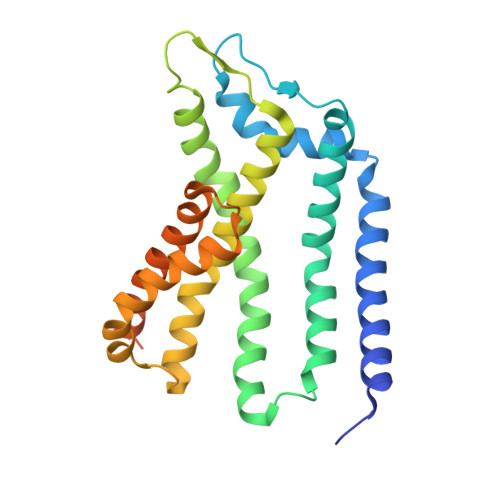 |
UniProt | |||||
Find proteins for P69423 (Escherichia coli (strain K12)) Explore P69423 Go to UniProtKB: P69423 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P69423 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glucans biosynthesis protein D | C [auth G], H, I | 552 | Escherichia coli | Mutation(s): 0 Gene Names: opgD, mdoD, BGM66_002243, BJI68_05615, CTR35_003307, E4K51_22440, E6D34_24965, FOI11_006015, FOI11_12985, FWK02_00580... | 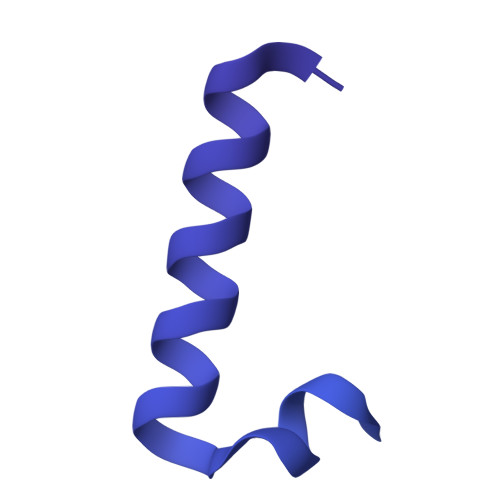 |
UniProt | |||||
Find proteins for P40120 (Escherichia coli (strain K12)) Explore P40120 Go to UniProtKB: P40120 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P40120 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Medical Research Council (MRC, United Kingdom) | United Kingdom | MR/L000776/1 |
| Wellcome Trust | United Kingdom | 107929/Z/15/Z |
| National Institutes of Health/National Cancer Institute (NIH/NCI) | United States | Intramural Research Program |