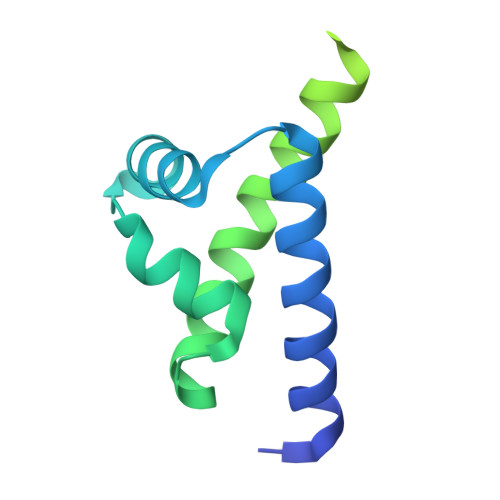
Coupled Heterogeneity to Dimeric Site-Specific Binding by the POU-Family Transcription Factor OCT2.
Terrell, J.R., Poon, G.M.K.(2025) J Phys Chem B 129: 2138-2148
- PubMed: 39960871
- DOI: https://doi.org/10.1021/acs.jpcb.4c07071
- Primary Citation of Related Structures:
9DZM - PubMed Abstract:
POU-family transcription factors regulate metazoan gene expression via a bipartite DNA-binding domain consisting of two covalently linked helix-turn-helix subdomains, POU S and POU H . POU factors bind as dimers to DNA half-sites to form complexes with a variable quaternary structure. To enhance the knowledge of the physical chemistry of dimeric POU/DNA recognition, we carried out a crystallographic and titration analysis of the cooperative homodimer formed by the POU factor OCT2 and an optimized palindromic DNA site known as MORE. The data evidence strong heterogeneity in the binding and formation of secondary complexes in site-specific DNA recognition by OCT2 at thermodynamic equilibrium. These secondary complexes are strictly contingent to the site-specific complex, detectable at subsaturating OCT2 concentrations, and cooperate with nonspecific binding to guide the affinity of the site-specific complex. Modulation with salt and poly[d(I-C)] unmasks the compensation between nonspecific DNA depleting unbound OCT2 on the one hand while driving specific binding by intermolecular transfer of OCT2 via secondary complexes on the other. Molecular dynamics simulations extend a mechanism, previously proposed for POU monomers, in which the two subdomains dynamically cross-link DNA strands to form supramolecular dimeric POU/DNA complexes at equilibrium.
- Department of Chemistry, Georgia State University, Atlanta, Georgia 30303, United States.
Organizational Affiliation: