Unveiling the hidden interactome of CRBN molecular glues.
Baek, K., Metivier, R.J., Roy Burman, S.S., Bushman, J.W., Yoon, H., Lumpkin, R.J., Ryan, J.K., Abeja, D.M., Lakshminarayan, M., Yue, H., Ojeda, S., Xiong, Y., Che, J., Verano, A.L., Schmoker, A.M., Gray, N.S., Donovan, K.A., Fischer, E.S.(2025) Nat Commun 16: 6831-6831
- PubMed: 40707481
- DOI: https://doi.org/10.1038/s41467-025-62099-w
- Primary Citation of Related Structures:
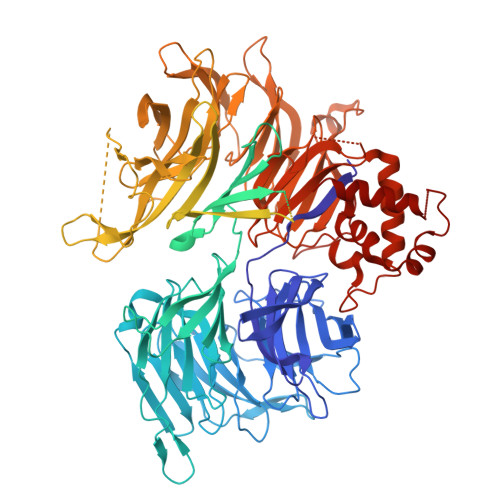
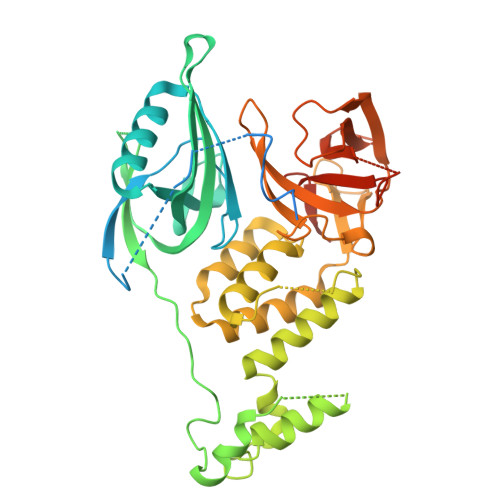
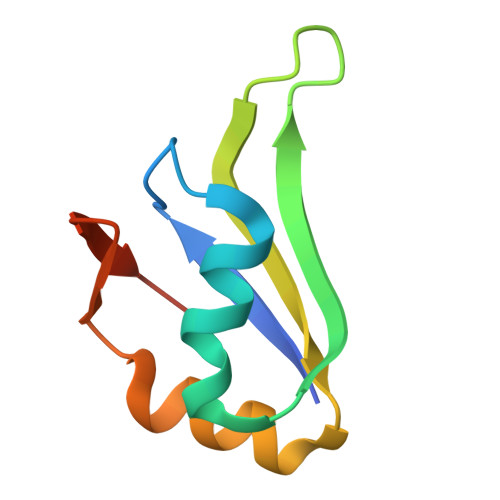
9DWV, 9DWW - PubMed Abstract:
Induced proximity by molecular glues refers to strategies that leverage the recruitment of proteins to facilitate their modification, regulation or degradation. As prospective design of molecular glues remains challenging, unbiased discovery methods are necessary to discover new chemical targets. Here we establish a high throughput affinity proteomics workflow leveraging E3 ligase activity-impaired CRBN-DDB1ΔB in cell lysates for the unbiased identification of molecular glue targets. By mapping the interaction landscape of CRBN-binding molecular glues, we unveil 298 protein targets and demonstrate the utility of enrichment methods for identifying targets overlooked by established methods. We use a computational workflow to estimate target confidence and perform biochemical and structural validation of uncharacterized neo-substrates. We further identify a lead compound for the previously untargeted non-zinc finger PPIL4 through a biochemical screen. Our study provides a comprehensive inventory of targets chemically recruited to CRBN and delivers a robust and scalable workflow for identifying drug-induced protein interactions in cell lysates.
- Department of Cancer Biology, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Organizational Affiliation: