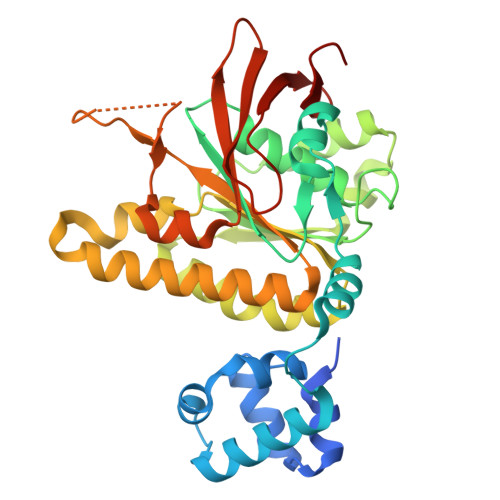
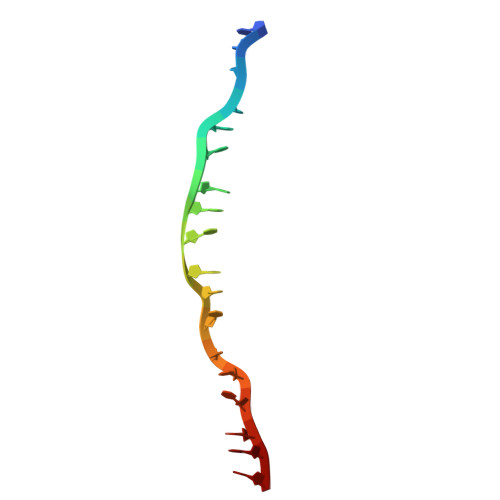
Lineage-specific amino acids define functional attributes of the protomer-protomer interfaces for the Rad51 and Dmc1 recombinases.
Petassi, M., Shin, Y., Jessop, A.M., Morse, K., Kim, S.Y., Kuppa, S., Matei, R., Raina, V.B., Greene, E.C.(2026) J Biological Chem 302: 111019-111019
- PubMed: 41371341
- DOI: https://doi.org/10.1016/j.jbc.2025.111019
- Primary Citation of Related Structures:
9D46, 9D4N - PubMed Abstract:
Most eukaryotes possess two Rad51/RecA family DNA recombinases that are thought to have arisen from an ancient gene duplication event: Rad51, which is expressed in both mitosis and meiosis; and Dmc1, which is only expressed in meiosis. To explore the evolutionary relationship between these recombinases, here, we present high-resolution cryo-EM structures of Saccharomyces cerevisiae Rad51 filaments and S. cerevisiae Dmc1 filaments bound to ssDNA, which reveal a pair of stacked interfacial aromatic amino acid residues that are nearly universally conserved in Rad51 but are absent from Dmc1. We use a combination of bioinformatics, genetic analysis of natural sequence variation, and deep mutational analysis to probe the functionally tolerated sequence space for these stacked aromatic residues. Our findings demonstrate that the functional landscape of the interfacial aromatic residues within the Rad51 filament is highly constrained. In contrast, the amino acids at the equivalent positions within the Dmc1 filament exhibit a broad functional landscape. This work helps highlight the distinct evolutionary trajectories of these two eukaryotic recombinases, which may have contributed to their functional and mechanistic divergence.
- Department of Biochemistry & Molecular Biophysics, Columbia University Irving Medical Center, New York, New York, USA.
Organizational Affiliation: