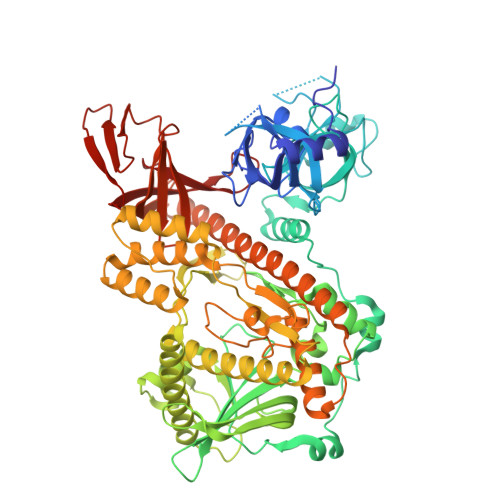
Structural and mechanistic insights into Dis3L2-mediated degradation of structured RNA.
Matos, R.G., Garg, A., Costa, S.M., Pereira, P., Arraiano, C.M., Joshua-Tor, L., Viegas, S.C.(2025) RNA 31: 1859-1871
- PubMed: 41033841
- DOI: https://doi.org/10.1261/rna.080685.125
- Primary Citation of Related Structures:
9CY7 - PubMed Abstract:
The RNase II/RNB family of exoribonucleases is present in all domains of life and includes three main eukaryotic members, the Dis3-like proteins (Dis3, Dis3L1, and Dis3L2). At the cellular level, Dis3L2 is distinguished by the unique preference for uridylated RNA substrates and the highest efficiency in degrading double-stranded RNA. Defects in these enzymes have been linked to some types of cancers and overgrowth disorders in humans. In this work, we used the Dis3L2 protein from the model organism Schizosaccharomyces pombe (SpDis3L2) to better understand the mechanism of action of Dis3-like exoribonucleases, and to elucidate how single amino acid substitutions in these proteins can affect the biochemical properties of the enzymes, potentially contributing to the molecular basis of the related human diseases. We determined the crystal structure of SpDis3L2 bound to a U 13 RNA, in which the protein displays a typical vase-like conformation, accommodating 6 nucleotides of the RNA 3'-end. Furthermore, we constructed two SpDis3L2 protein variants, harboring single amino acid substitutions mimicking the ones already found in human patients, to test their catalytic activity in vitro. We highlight the A756R SpDis3L2 variant, which loses the ability to degrade double-stranded RNA substrates and accumulates intermediate degradation products when degrading single-stranded RNA substrates. As such, A756 seems to be a key residue responsible for the normal cellular function of Dis3L2, specifically regarding its important role in the degradation of structured RNA substrates.
- Control of Gene Expression Laboratory, Instituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, 2780-157 Oeiras, Portugal.
Organizational Affiliation: