Structural basis for nucleolin recognition of MYC promoter G-quadruplex.
Chen, L., Dickerhoff, J., Zheng, K.W., Erramilli, S., Feng, H., Wu, G., Onel, B., Chen, Y., Wang, K.B., Carver, M., Lin, C., Sakai, S., Wan, J., Vinson, C., Hurley, L., Kossiakoff, A.A., Deng, N., Bai, Y., Noinaj, N., Yang, D.(2025) Science 388: eadr1752-eadr1752
- PubMed: 40245140
- DOI: https://doi.org/10.1126/science.adr1752
- Primary Citation of Related Structures:
9CB5 - PubMed Abstract:
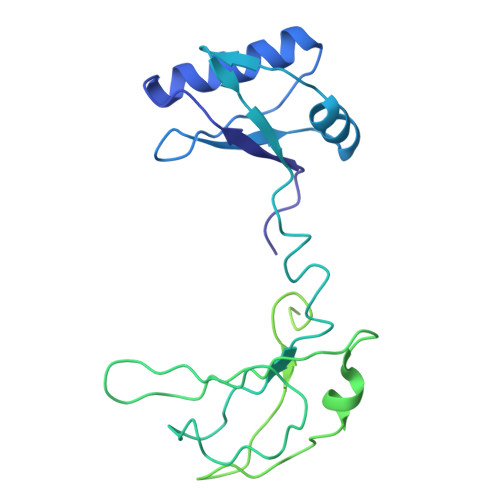
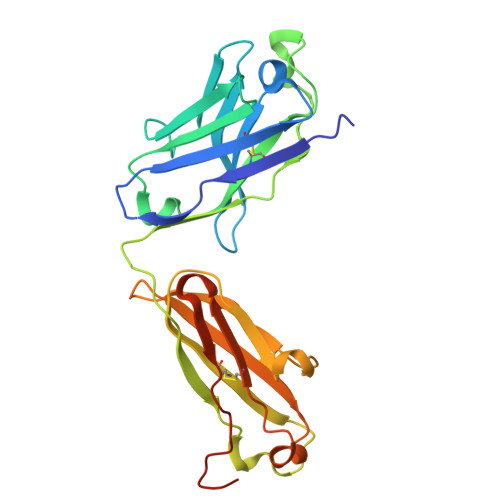
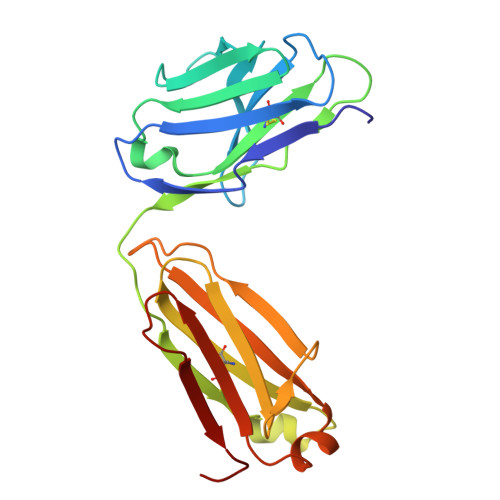
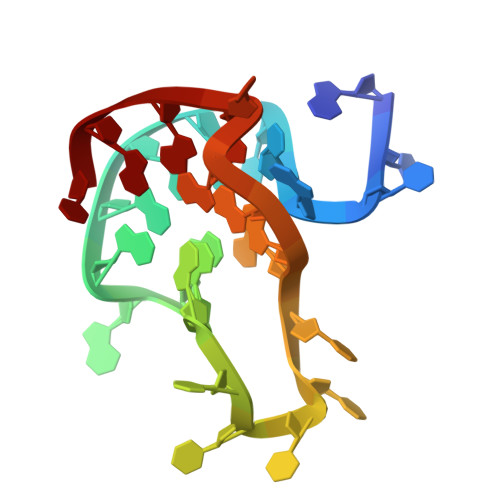
The MYC oncogene promoter G-quadruplex (MycG4) regulates transcription and is a prevalent G4 locus in immortal cells. Nucleolin, a major MycG4-binding protein, exhibits greater affinity for MycG4 than for nucleolin recognition element (NRE) RNA. Nucleolin's four RNA binding domains (RBDs) are essential for high-affinity MycG4 binding. We present the 2.6-angstrom crystal structure of the nucleolin-MycG4 complex, revealing a folded parallel three-tetrad G-quadruplex with two coordinating potassium ions (K + ), interacting with RBD1, RBD2, and Linker12 through its 6-nucleotide (nt) central loop and 5' flanking region. RBD3 and RBD4 bind MycG4's 1-nt loops as demonstrated by nuclear magnetic resonance (NMR). Cleavage under targets and tagmentation sequencing confirmed nucleolin's binding to MycG4 in cells. Our results revealed a G4 conformation-based recognition by a regulating protein through multivalent interactions, suggesting that G4s are nucleolin's primary cellular substrates, indicating G4 epigenetic transcriptional regulation and helping G4-targeted drug discovery.
- Borch Department of Medicinal Chemistry and Molecular Pharmacology, College of .Pharmacy, Purdue University, West Lafayette, IN, USA.
Organizational Affiliation: