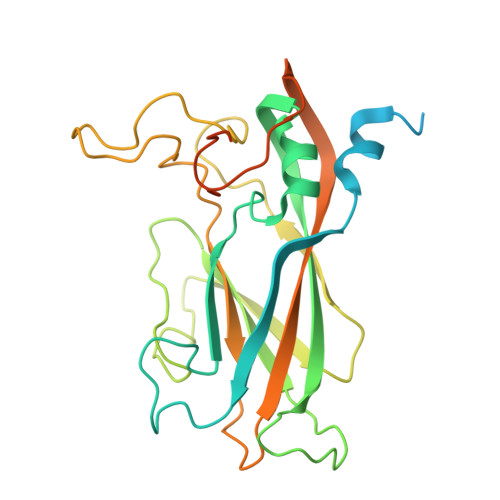
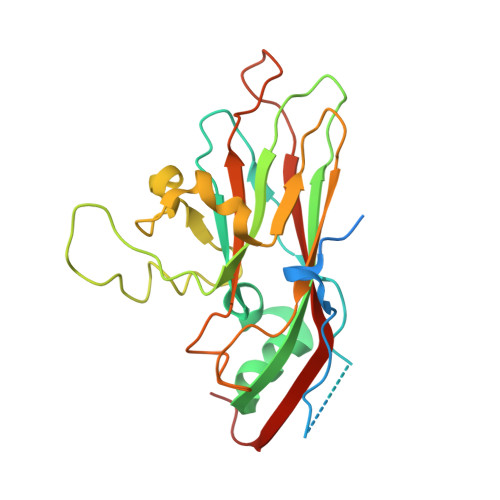
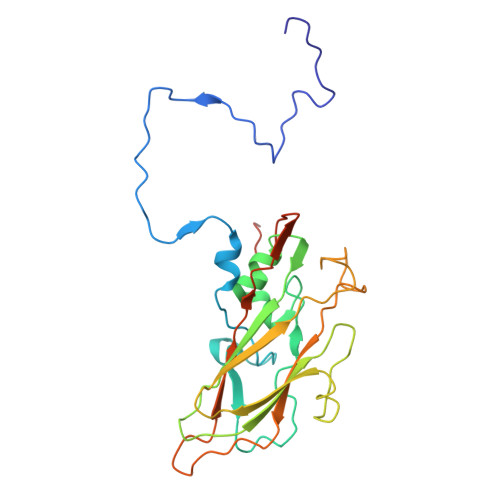
Structural insights from vaccine candidates for EV-D68.
Cheng, J., Krug, P.W., Lei, H., Moss, D.L., Lang, Z.C., Morton, A.J., Shen, C.H., Pletnev, S., Huang, R.K., Pierson, T.C., Zhou, T., Ruckwardt, T.J., Kwong, P.D.(2025) Commun Biol 8: 860-860
- PubMed: 40467847
- DOI: https://doi.org/10.1038/s42003-025-08253-y
- Primary Citation of Related Structures:
9C3J, 9C4A, 9C8F, 9C8G, 9C8H, 9C8I - PubMed Abstract:
Enterovirus D68 (EV-D68), a member of the Picornaviridae family, causes respiratory illness and can lead to acute flaccid myelitis in children. No specific treatment or vaccine is available. Here, we determine cryo-EM structures of EV-D68 virus-like particles (VLPs), inactivated virus particles (InVPs), and altered virus particles (A-particles) from B3 and A2 subclades. The B3 VLP is a current vaccine candidate, which we show closely resembles its InVP counterpart, particularly at the 5-fold axis of symmetry, the target of potent neutralizing antibodies. Similar structural conservation was observed in the A2 subclade. Sequence variation between B3 and A2 mainly occurred in flexible loops displayed on the particle surface. A canyon-filling pocket factor was present in B3 InVP but absent in A2 InVP. A-particles were predominant in β-propiolactone-inactivated virus at longer but not shorter incubation. Overall, our findings highlight EV-D68 similarities and subclade-specific differences, offering structural insights that relate to vaccine development.
- Vaccine Research Center, NIAID, National Institutes of Health, Bethesda, MD, 20892, USA.
Organizational Affiliation: