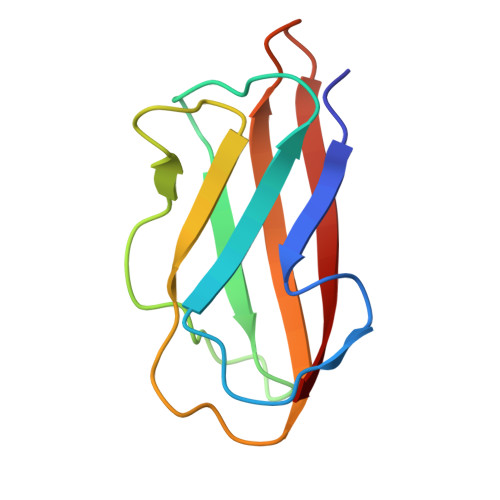
Copper acquisition in Bacillus subtilis involves Cu(II) exchange between YcnI and YcnJ.
de Oliveira Silva, Y.R., Barnes, G., Zheng, D., Zhitnitsky, D., Geathers, S.J., Peters, S.C., Szalai, V.A., Helmann, J.D., Fisher, O.S.(2025) J Biological Chem 301: 110480-110480
- PubMed: 40669668
- DOI: https://doi.org/10.1016/j.jbc.2025.110480
- Primary Citation of Related Structures:
9C14 - PubMed Abstract:
The transition metal copper is biologically essential across all three domains of life. Several copper-dependent proteins and enzymes produced by the Gram-positive bacterium Bacillus subtilis have been characterized. However, many questions remain about how copper is recognized and trafficked to metalate cuproproteins. The ycnKJI operon in B. subtilis encodes a suite of proteins implicated in copper uptake and regulation, including the copper-binding protein YcnI and the putative copper importer YcnJ. Here, we demonstrate that one of the extracellular domains within YcnJ (YcnJ CopC ) binds Cu(II) in 1:1 stoichiometry with high affinity using a histidine brace motif. Biochemical results reveal that YcnJ CopC and YcnI can exchange Cu(II). Genetic studies reveal that loss of either YcnI or YcnJ, or mutation of the key residues required for Cu(II)-binding, leads to a growth defect under conditions of copper limitation. Together, these data suggest that the Cu(II)-binding sites in both YcnI and YcnJ may contribute to efficient import under Cu limited conditions. Our results support a model in which YcnI may sequester Cu(II) from YcnJ, serving a regulatory role to limit the amount of copper that enters the cytoplasm and allowing Cu(II) to be stored for later import on the outer face of the membrane. This transfer of Cu(II) between extracellular domains of membrane-bound proteins represents a potential new paradigm in bacterial copper usage.
- Department of Chemistry, Lehigh University, 6 E Packer Ave, Bethlehem, PA, USA, 18015.
Organizational Affiliation: