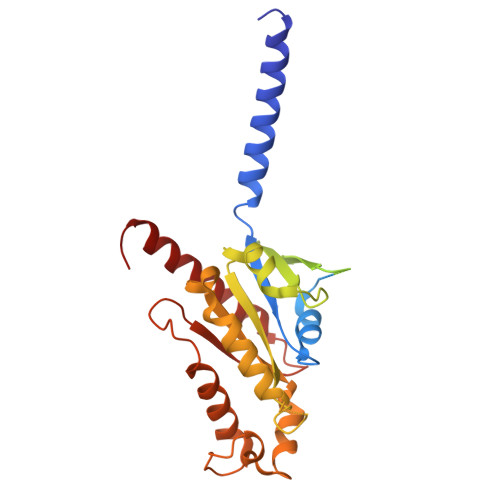
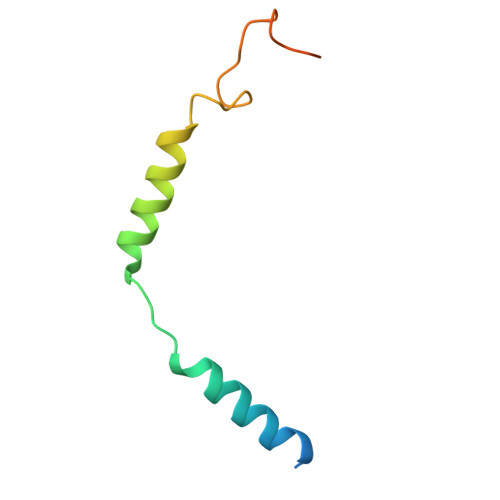
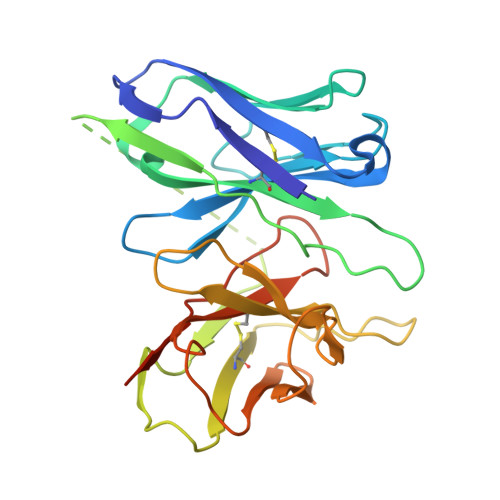
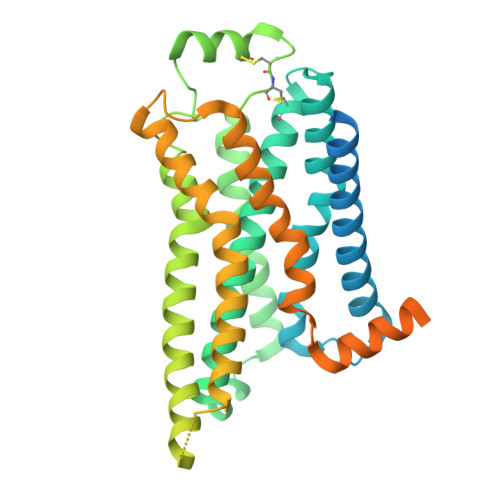
Structure and dynamics determine G protein coupling specificity at a class A GPCR.
Casiraghi, M., Wang, H., Brennan, P.C., Habrian, C., Hubner, H., Schmidt, M.F., Maul, L., Pani, B., Bahriz, S.M.F.M., Xu, B., Staffen, N., Assafa, T.E., Chen, B., White, E., Sunahara, R.K., Inoue, A., Xiang, Y.K., Lefkowitz, R.J., Isacoff, E.Y., Nucci, N., Gmeiner, P., Lerch, M.T., Kobilka, B.K.(2025) Sci Adv 11: eadq3971-eadq3971
- PubMed: 40106559
- DOI: https://doi.org/10.1126/sciadv.adq3971
- Primary Citation of Related Structures:
9BUY - PubMed Abstract:
G protein-coupled receptors (GPCRs) exhibit varying degrees of selectivity for different G protein isoforms. Despite the abundant structures of GPCR-G protein complexes, little is known about the mechanism of G protein coupling specificity. The β 2 -adrenergic receptor is an example of GPCR with high selectivity for Gαs, the stimulatory G protein for adenylyl cyclase, and much weaker for the Gαi family of G proteins inhibiting adenylyl cyclase. By developing a Gαi-biased agonist (LM189), we provide structural and biophysical evidence supporting that distinct conformations at ICL2 and TM6 are required for coupling of the different G protein subtypes Gαs and Gαi. These results deepen our understanding of G protein specificity and bias and can accelerate the design of ligands that select for preferred signaling pathways.
- Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA, USA.
Organizational Affiliation: