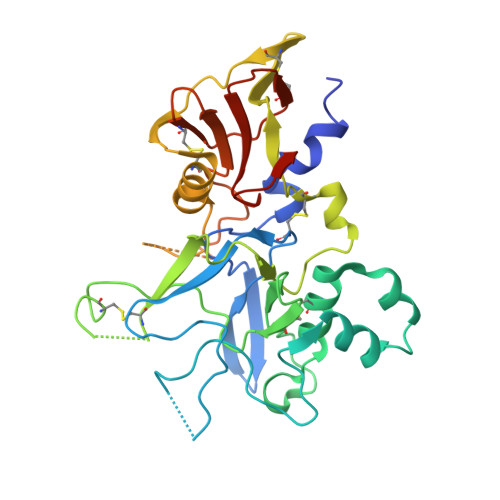
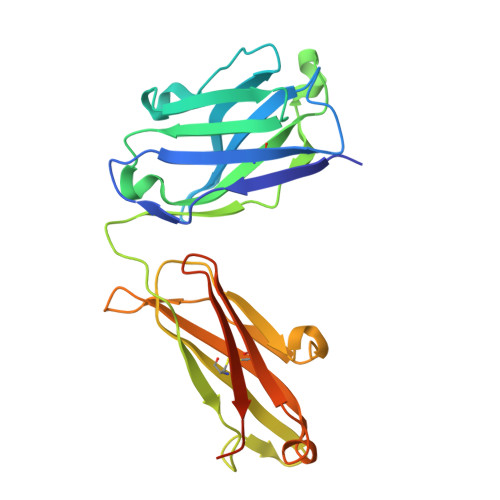
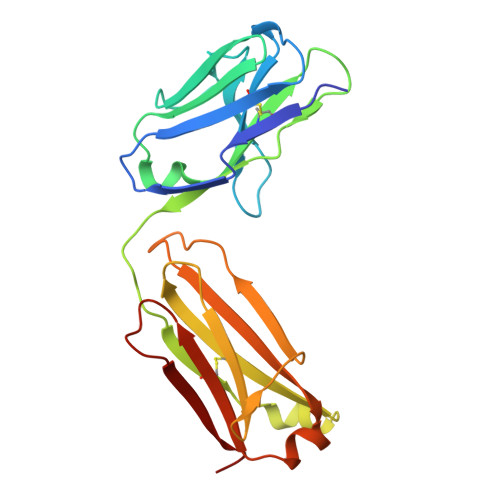
A strain-transcending anti-AMA1 human monoclonal antibody neutralizes malaria parasites independent of direct RON2L receptor blockade.
Patel, P.N., Diouf, A., Dickey, T.H., Tang, W.K., Hopp, C.S., Traore, B., Long, C.A., Miura, K., Crompton, P.D., Tolia, N.H.(2025) Cell Rep Med 6: 101985-101985
- PubMed: 40020675
- DOI: https://doi.org/10.1016/j.xcrm.2025.101985
- Primary Citation of Related Structures:
9BJG, 9BJH - PubMed Abstract:
Plasmodium falciparum apical membrane antigen 1 (AMA1) binds a loop in rhoptry neck protein 2 (RON2L) during red cell invasion and is a target for vaccines and therapeutic antibodies against malaria. Here, we report a panel of AMA1-specific naturally acquired human monoclonal antibodies (hmAbs) derived from individuals living in malaria-endemic regions. Two neutralizing hmAbs engage AMA1 independent of the RON2L-binding site. The hmAb 75B10 demonstrates potent strain-transcending neutralization that is independent of RON2L blockade, emphasizing that epitopes outside the RON2L-binding site elicit broad protection against variant parasite strains. The combination of these hmAbs synergistically enhances parasite neutralization. Vaccination with a structure-based design (SBD1) that mimics the AMA1-RON2L complex elicited antibodies similar to the two neutralizing hmAbs connecting vaccination to naturally acquired immunity in humans. The structural definition of a strain-transcending epitope on AMA1 targeted by naturally acquired hmAb establishes paradigms for developing AMA1-based vaccines and therapeutic antibodies.
- Host-Pathogen Interactions and Structural Vaccinology Section, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Organizational Affiliation: