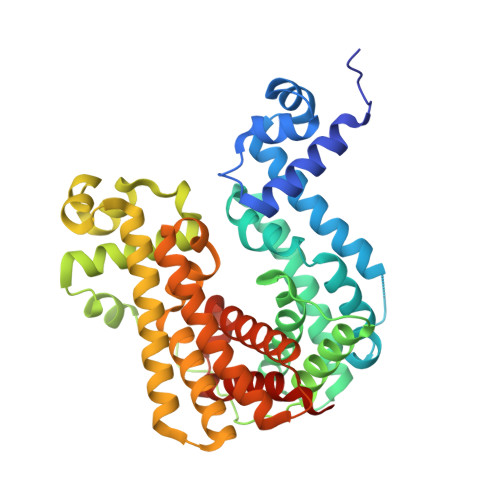
Mycobacterium tuberculosis Mce3R TetR-like Repressor Forms an Asymmetric Four-Helix Bundle and Binds a Nonpalindrome Sequence†.
Panagoda, N.T., Balazsi, G., Sampson, N.S.(2024) ACS Chem Biol 19: 2580-2592
- PubMed: 39545866
- DOI: https://doi.org/10.1021/acschembio.4c00687
- Primary Citation of Related Structures:
9B7Y - PubMed Abstract:
Mycobacterium tuberculosis ( Mtb ), the causative agent of tuberculosis, is a major global health concern. TetR family repressors (TFRs) are important for Mtb 's adaptation to the human host environment. Our study focuses on one notable Mtb repressor, Mce3R, composed of an unusual double TFR motif. Mce3R-regulated genes encode enzymes implicated in cholesterol metabolism, resistance against reactive oxygen species, and lipid transport activities important for Mtb survival and persistence in the host and for the cellular activity of a 6-azasteroid derivative. Here, we present the structure of Mce3R bound to its DNA operator, unveiling a unique asymmetric assembly previously unreported. We obtained a candidate DNA-binding motif through MEME motif analysis, comparing intergenic regions of mce3R orthologues and identifying nonpalindromic regions conserved between orthologues. Using an electrophoretic mobility shift assay (EMSA), we confirmed that Mce3R binds to a 123-bp sequence that includes the predicted motif. Using scrambled DNA and DNA oligonucleotides of varying lengths with sequences from the upstream region of the yrbE3A ( mce3 ) operon, we elucidated the operator region to be composed of two Mce3R binding sites, each a 25-bp asymmetric sequence separated by 53 bp. Mce3R binds with a higher affinity to the downstream site with a K d of 2.4 ± 0.7 nM. The cryo-EM structure of Mce3R bound to the 123-bp sequence was refined to a resolution of 2.51 Å. Each Mce3R monomer comprises 21 α-helices (α1-α21) folded into an asymmetric TFR-like structure with a core asymmetric four-helix bundle. This complex has two nonidentical HTH motifs and a single ligand-binding domain. The two nonidentical HTHs from each TFR bind within the high-affinity, nonpalindromic operator motif, with Arg53 and Lys262 inserted into the major groove. Site-directed mutagenesis of Arg53 to alanine abrogated DNA binding, validating the Mce3R/DNA structure obtained. Among 811,645 particles, 63% were Mce3R homodimer bound to two duplex oligonucleotides. Mce3R homodimerizes primarily through α15, and each monomer binds to an identical site in the DNA duplex oligonucleotide.
- Department of Chemistry, Stony Brook University, Stony Brook, New York 11794-3400, United States.
Organizational Affiliation: