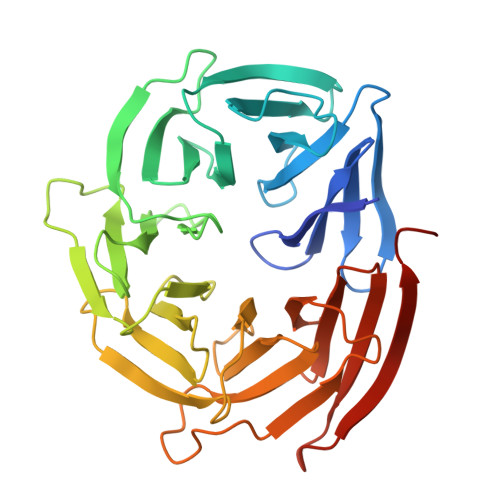
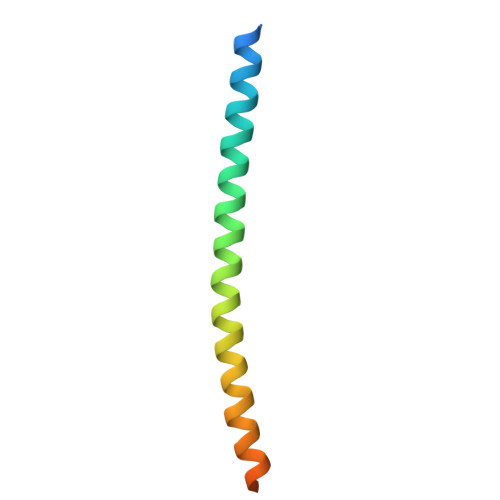
Structure of the WIPI3/ATG16L1 Complex Reveals the Molecular Basis for the Recruitment of the ATG12~ATG5-ATG16L1 Complex by WIPI3.
Gong, X., Wang, Y., Zhou, Y., Pan, L.(2024) Cells 13
- PubMed: 39768203
- DOI: https://doi.org/10.3390/cells13242113
- Primary Citation of Related Structures:
8ZQG - PubMed Abstract:
Macroautophagy deploys a wealth of autophagy-related proteins to synthesize the double-membrane autophagosome, in order to engulf cytosolic components for lysosome-dependent degradation. The recruitment of the ATG12~ATG5-ATG16L1 complex by WIPI family proteins is a crucial step in autophagosome formation. Nevertheless, the molecular mechanism by which WIPI3 facilitates the recruitment of the ATG12~ATG5-ATG16L1 complex remains largely unknown. Here, we uncover that WIPI3 can directly interact with the coiled-coil domain of ATG16L1. By determining the crystal structure of WIPI3 in complex with ATG16L1 coiled-coil, we elucidate the molecular basis underpinning the specific recruitment of the ATG12~ATG5-ATG16L1 complex by WIPI3. Moreover, we demonstrate that WIPI2 and WIPI3 are competitive for interacting with ATG16L1 coiled-coil, and ATG16L1 and ATG2 are mutually exclusive in binding to WIPI3. In all, our findings provide mechanistic insights into the WIPI3/ATG16L1 interaction, and are valuable for further understanding the activation mechanism of the ATG12~ATG5-ATG16L1 complex as well as the working mode of WIPI3 in autophagy.
- State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200032, China.
Organizational Affiliation: