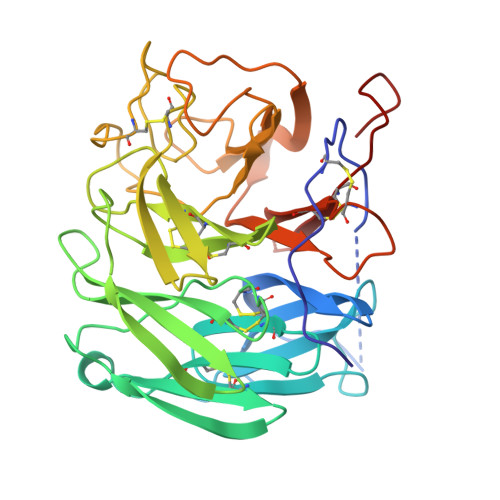
A human monoclonal antibody targeting the monomeric N6 neuraminidase confers protection against avian H5N6 influenza virus infection.
Wang, M., Gao, Y., Shen, C., Yang, W., Peng, Q., Cheng, J., Shen, H.M., Yang, Y., Gao, G.F., Shi, Y.(2024) Nat Commun 15: 8871-8871
- PubMed: 39402031
- DOI: https://doi.org/10.1038/s41467-024-53301-6
- Primary Citation of Related Structures:
8Z4E - PubMed Abstract:
The influenza neuraminidase (NA) is a potential target for the development of a next-generation influenza vaccine, but its antigenicity is not well understood. Here, we isolate an anti-N6 human monoclonal antibody, named 18_14D, from an H5N6 avian influenza virus (AIV) infected patient. The antibody weakly inhibits enzymatic activity but confers protection in female mice, mainly via ADCC function. The cryo-EM structure shows that 18_14D binds to a unique epitope on the lateral surface of the N6 tetramer, preventing the formation of tightly closed NA tetramers. These findings contribute to the molecular understanding of protective immune responses to NA of AIVs in humans and open an avenue for the rational design of NA-based vaccines.
- CAS Key Laboratory of Pathogen Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences (CAS), Beijing, China.
Organizational Affiliation: