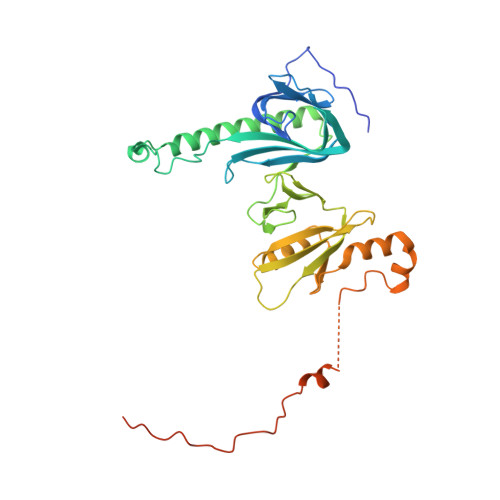
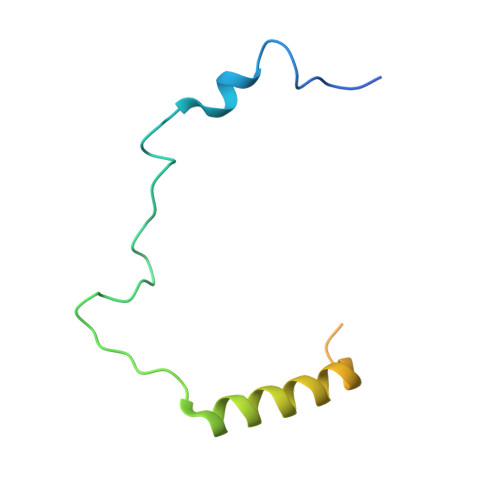
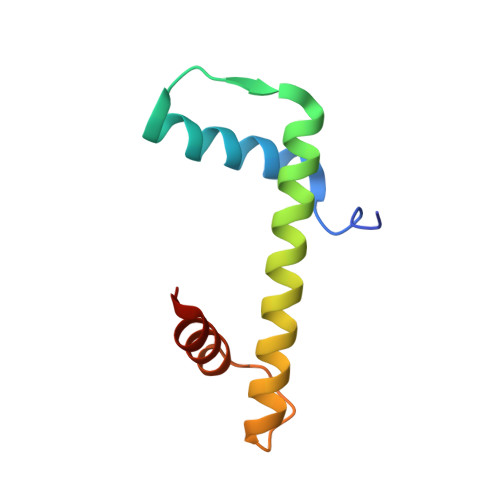
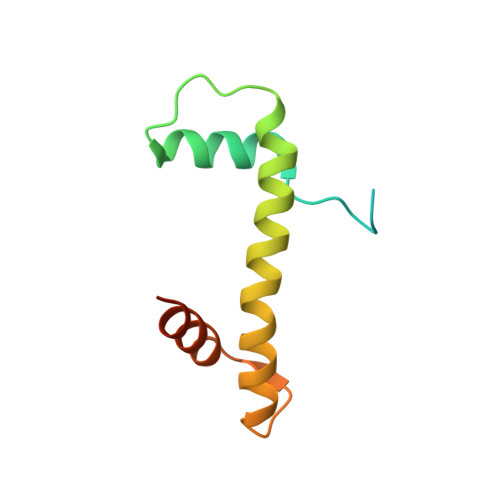
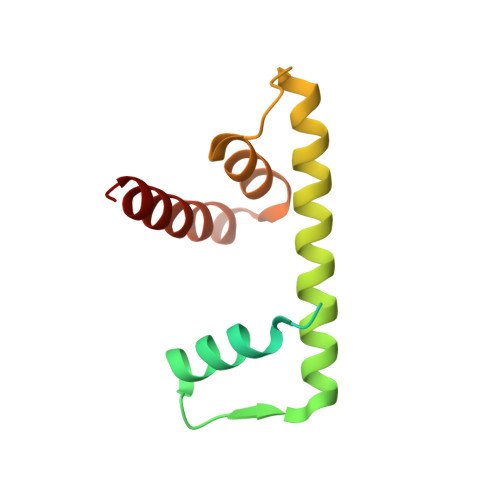
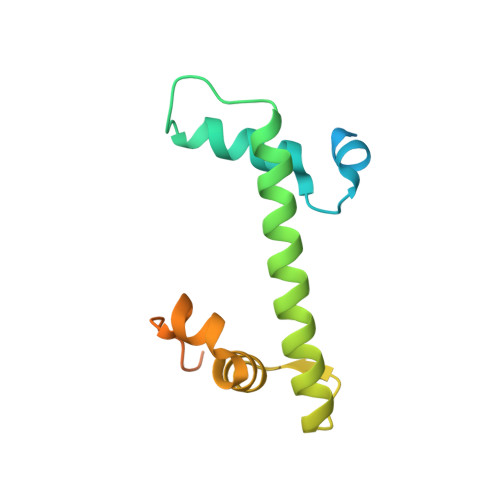
Structure of a histone hexamer bound by the chaperone domains of SPT16 and MCM2.
Gan, S., Yang, W.S., Wei, L., Zhang, Z., Xu, R.M.(2024) Sci China Life Sci 67: 1305-1307
- PubMed: 38478295
- DOI: https://doi.org/10.1007/s11427-024-2560-8
- Primary Citation of Related Structures:
8YJF, 8YJM - Key Laboratory of Epigenetic Regulation and Intervention, Institute of Biophysics, Chinese Academy of Sciences, Beijing, 100101, China.
Organizational Affiliation: