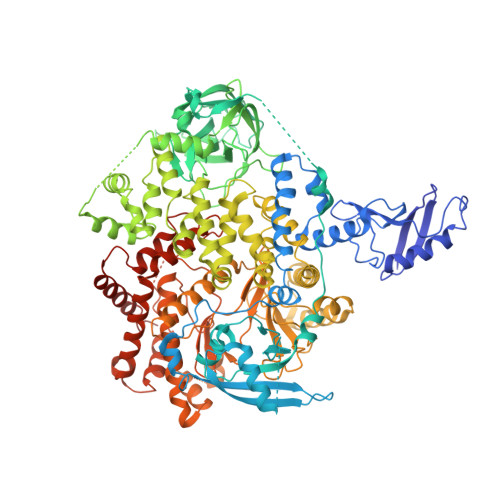
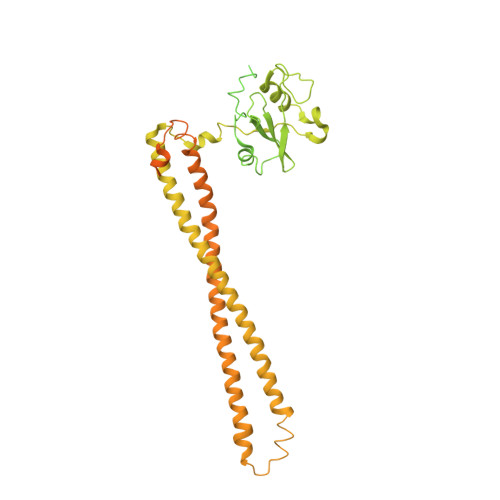
Cryo-EM structures reveal two allosteric inhibition modes of PI3K alpha H1047R involving a re-shaping of the activation loop.
Huang, X., Wang, K., Han, J., Chen, X., Wang, Z., Wu, T., Yu, B., Zhao, F., Wang, X., Li, H., Xie, Z., Zhu, X., Zhong, W., Ren, X.(2024) Structure 32: 907-917.e7
- PubMed: 38582077
- DOI: https://doi.org/10.1016/j.str.2024.03.007
- Primary Citation of Related Structures:
8W9A, 8W9B - PubMed Abstract:
PI3Kα is a lipid kinase that phosphorylates PIP2 and generates PIP3. The hyperactive PI3Kα mutation, H1047R, accounts for about 14% of breast cancer, making it a highly attractive target for drug discovery. Here, we report the cryo-EM structures of PI3Kα H1047R bound to two different allosteric inhibitors QR-7909 and QR-8557 at a global resolution of 2.7 Å and 3.0 Å, respectively. The structures reveal two distinct binding pockets on the opposite sides of the activation loop. Structural and MD simulation analyses show that the allosteric binding of QR-7909 and QR-8557 inhibit PI3Kα H1047R hyper-activity by reducing the fluctuation and mobility of the activation loop. Our work provides a strong rational basis for a further optimization and development of highly selective drug candidates to treat PI3Kα H1047R -driven cancers.
- Regor Therapeutics Group, Shanghai 201210, China. Electronic address: xiuliang.huang@qlregor.com.
Organizational Affiliation: