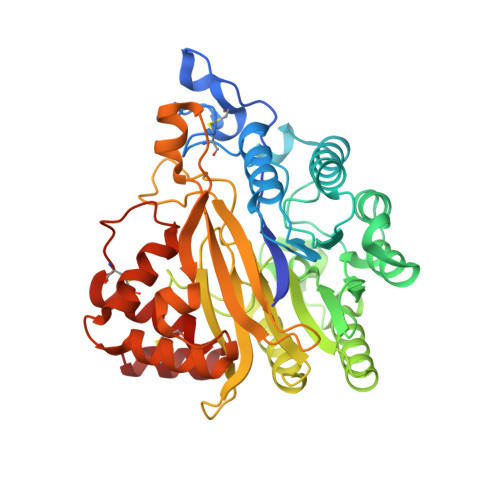
SMPDL3A is a cGAMP-degrading enzyme induced by LXR-mediated lipid metabolism to restrict cGAS-STING DNA sensing.
Hou, Y., Wang, Z., Liu, P., Wei, X., Zhang, Z., Fan, S., Zhang, L., Han, F., Song, Y., Chu, L., Zhang, C.(2023) Immunity 56: 2492-2507.e10
- PubMed: 37890481
- DOI: https://doi.org/10.1016/j.immuni.2023.10.001
- Primary Citation of Related Structures:
8W6P, 8W6R - PubMed Abstract:
Lipid metabolism has been associated with the cyclic guanosine monophosphate (GMP)-AMP synthase (cGAS) stimulator of interferon genes (STING) DNA-sensing pathway, but our understanding of how these signals are integrated into a cohesive immunometabolic program is lacking. Here, we have identified liver X receptor (LXR) agonists as potent inhibitors of STING signaling. We show that stimulation of lipid metabolism by LXR agonists specifically suppressed cyclic GMP-AMP (cGAMP)-STING signaling. Moreover, we developed cyclic dinucleotide-conjugated beads to biochemically isolate host effectors for cGAMP inhibition, and we found that LXR ligands stimulated the expression of sphingomyelin phosphodiesterase acid-like 3A (SMPDL3A), which is a 2'3'-cGAMP-degrading enzyme. Results of crystal structures suggest that cGAMP analog induces dimerization of SMPDL3A, and the dimerization is critical for cGAMP degradation. Additionally, we have provided evidence that SMPDL3A cleaves cGAMP to restrict STING signaling in cell culture and mouse models. Our results reveal SMPDL3A as a cGAMP-specific nuclease and demonstrate a mechanism for how LXR-associated lipid metabolism modulates STING-mediated innate immunity.
- State Key Laboratory of Membrane Biology, School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China.
Organizational Affiliation: