Cryo-EM structure of Escherichia coli Str K12 FtsE(E163Q)X/EnvC complex with ATP in peptidisc
Li, J., Xu, X., He, Y., Luo, M.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cell division ATP-binding protein FtsE | A [auth B], D | 222 | Escherichia coli K-12 | Mutation(s): 1 Gene Names: ftsE Membrane Entity: Yes | 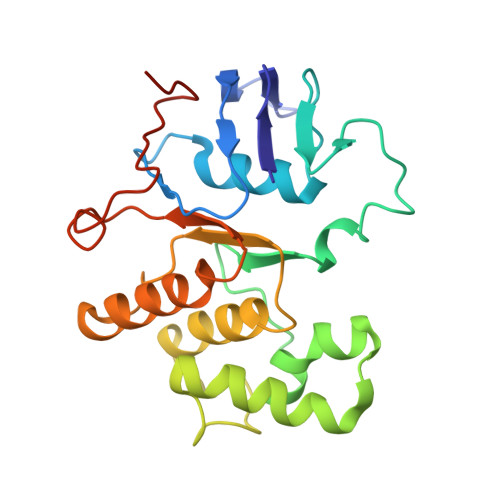 |
UniProt | |||||
Find proteins for P0A9R7 (Escherichia coli (strain K12)) Explore P0A9R7 Go to UniProtKB: P0A9R7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0A9R7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cell division protein FtsX | B [auth A], C | 352 | Escherichia coli K-12 | Mutation(s): 0 Gene Names: ftsX Membrane Entity: Yes | 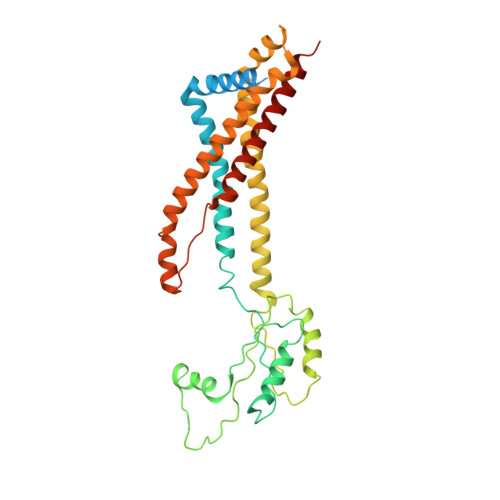 |
UniProt | |||||
Find proteins for P0AC30 (Escherichia coli (strain K12)) Explore P0AC30 Go to UniProtKB: P0AC30 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0AC30 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Murein hydrolase activator EnvC | 419 | Escherichia coli K-12 | Mutation(s): 0 Gene Names: envC Membrane Entity: Yes | 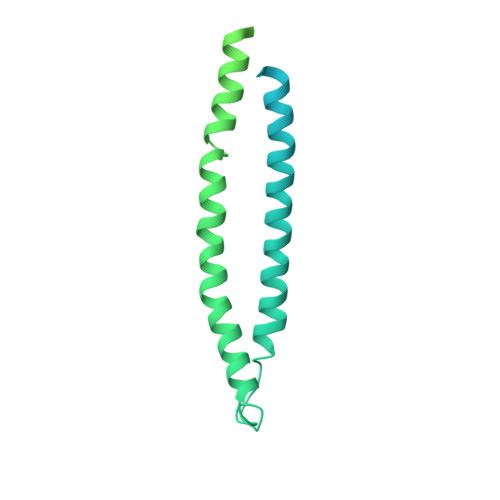 | |
UniProt | |||||
Find proteins for P37690 (Escherichia coli (strain K12)) Explore P37690 Go to UniProtKB: P37690 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P37690 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ATP (Subject of Investigation/LOI) Query on ATP | F [auth B], G [auth D] | ADENOSINE-5'-TRIPHOSPHATE C10 H16 N5 O13 P3 ZKHQWZAMYRWXGA-KQYNXXCUSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Ministry of Education (MoE, Singapore) | Singapore | A-0008412-00-00 |