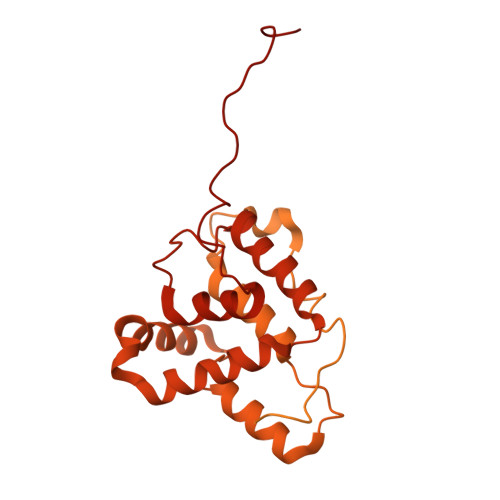
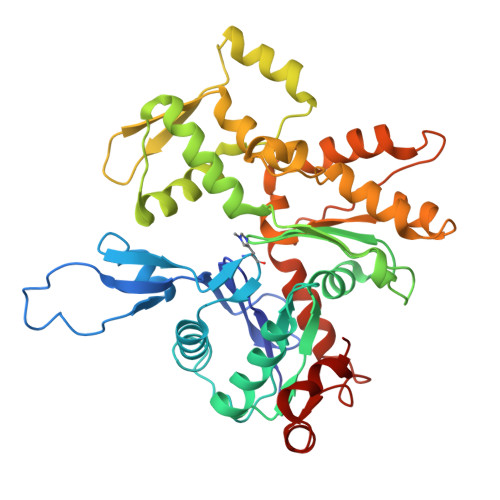
Cryo-EM structure of the bacterial effector protein SipA bound to F-actin reveals a unique mechanism for filament stabilization.
Guo, E., Chou, S.Z., Lara-Tejero, M., Galan, J.E.(2024) bioRxiv
- PubMed: 38187563
- DOI: https://doi.org/10.1101/2023.12.21.572903
- Primary Citation of Related Structures:
8VFM - PubMed Abstract:
The bacterial pathogen Salmonella spp. modulates cellular processes by delivering effector proteins through its type III secretion systems. Among these effectors, SipA facilitates bacterial invasion and promotes intestinal inflammation. The mechanisms by which this effector carries out these functions are incompletely understood although SipA's ability to modulate actin dynamics is central to some of these activities. Here we report the cryo-EM structure of SipA bound to filamentous actin. We show that this effector stabilizes actin filaments through unique interactions of its carboxy terminal domain with four actin subunits. Furthermore, our structure-function studies revealed that SipA's actin-binding activity is independent from its ability to stimulate intestinal inflammation. Overall, these studies illuminate critical aspects of Salmonella pathogenesis, and provide unique insight into the mechanisms by which a bacterial effector modulates actin dynamics.