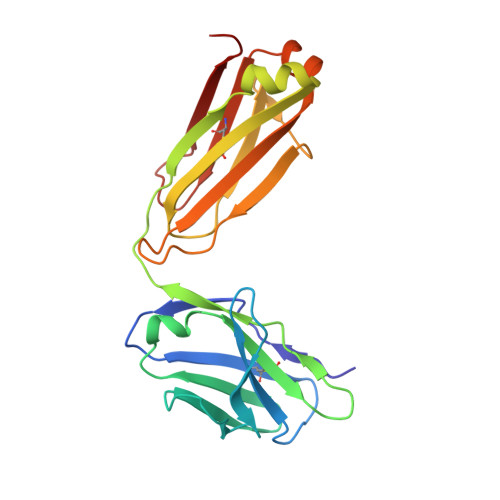
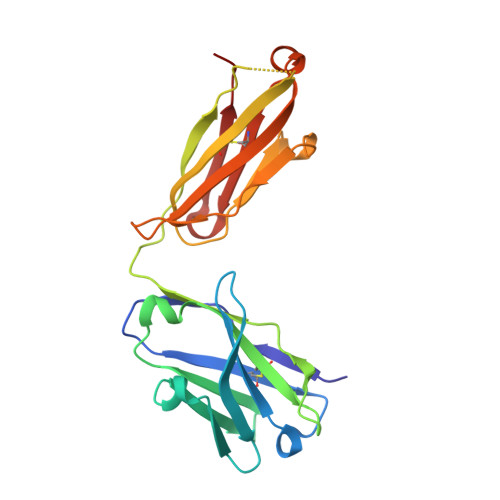
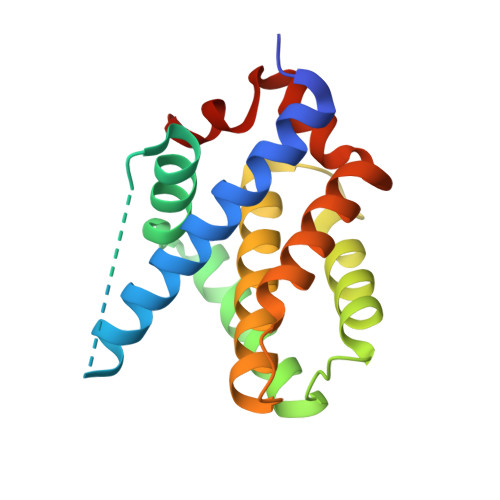
A novel inhibitory BAK antibody enables assessment of non-activated BAK in cancer cells.
Subas Satish, H.P., Iyer, S., Shi, M.X., Wong, A.W., Fischer, K.C., Wardak, A.Z., Lio, D., Brouwer, J.M., Uren, R.T., Czabotar, P.E., Miller, M.S., Kluck, R.M.(2024) Cell Death Differ 31: 711-721
- PubMed: 38582955
- DOI: https://doi.org/10.1038/s41418-024-01289-3
- Primary Citation of Related Structures:
8UKY - PubMed Abstract:
BAX and BAK are pro-apoptotic members of the BCL2 family that are required to permeabilize the mitochondrial outer membrane. The proteins can adopt a non-activated monomeric conformation, or an activated conformation in which the exposed BH3 domain facilitates binding either to a prosurvival protein or to another activated BAK or BAX protein to promote pore formation. Certain cancer cells are proposed to have high levels of activated BAK sequestered by MCL1 or BCLX L , thus priming these cells to undergo apoptosis in response to BH3 mimetic compounds that target MCL1 or BCLX L . Here we report the first antibody, 14G6, that is specific for the non-activated BAK conformer. A crystal structure of 14G6 Fab bound to BAK revealed a binding site encompassing both the α1 helix and α5-α6 hinge regions of BAK, two sites involved in the unfolding of BAK during its activation. In mitochondrial experiments, 14G6 inhibited BAK unfolding triggered by three diverse BAK activators, supporting crucial roles for both α1 dissociation and separation of the core (α2-α5) and latch (α6-α9) regions in BAK activation. 14G6 bound the majority of BAK in several leukaemia cell lines, and binding decreased following treatment with BH3 mimetics, indicating only minor levels of constitutively activated BAK in those cells. In summary, 14G6 provides a new means of assessing BAK status in response to anti-cancer treatments.
- Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, VIC, 3052, Australia.
Organizational Affiliation: