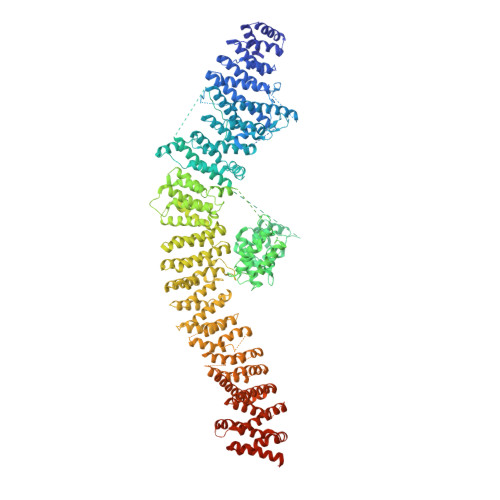
Sec7 regulatory domains scaffold autoinhibited and active conformations.
Brownfield, B.A., Richardson, B.C., Halaby, S.L., Fromme, J.C.(2024) Proc Natl Acad Sci U S A 121: e2318615121-e2318615121
- PubMed: 38416685
- DOI: https://doi.org/10.1073/pnas.2318615121
- Primary Citation of Related Structures:
8UCQ - PubMed Abstract:
The late stages of Golgi maturation involve a series of sequential trafficking events in which cargo-laden vesicles are produced and targeted to multiple distinct subcellular destinations. Each of these vesicle biogenesis events requires activation of an Arf GTPase by the Sec7/BIG guanine nucleotide exchange factor (GEF). Sec7 localization and activity is regulated by autoinhibition, positive feedback, and interaction with other GTPases. Although these mechanisms have been characterized biochemically, we lack a clear picture of how GEF localization and activity is modulated by these signals. Here, we report the cryogenic electron microscopy structure of full-length Sec7 in its autoinhibited form, revealing the architecture of its multiple regulatory domains. We use functional experiments to determine the basis for autoinhibition and use structural predictions to produce a model for an active conformation of the GEF that is supported empirically. This study therefore elucidates the conformational transition that Sec7 undergoes to become active on the organelle membrane surface.
- Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853.
Organizational Affiliation: