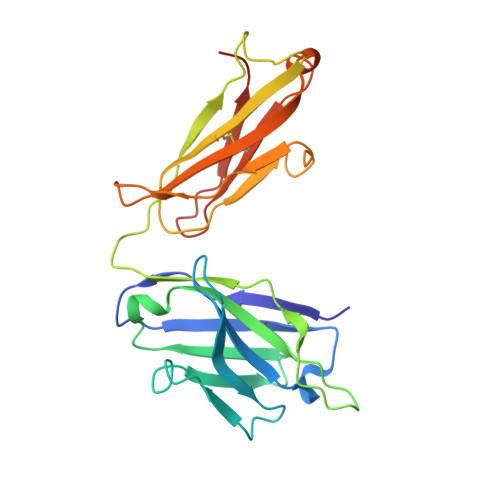
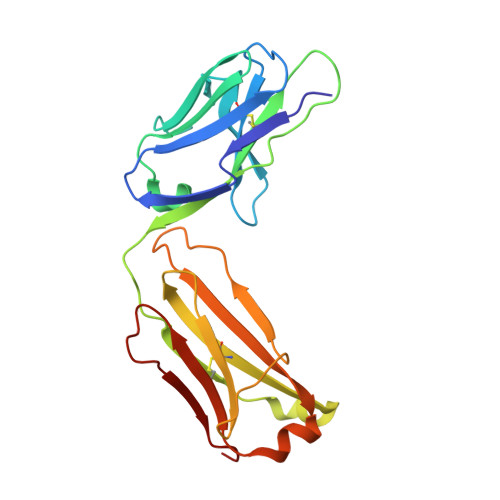
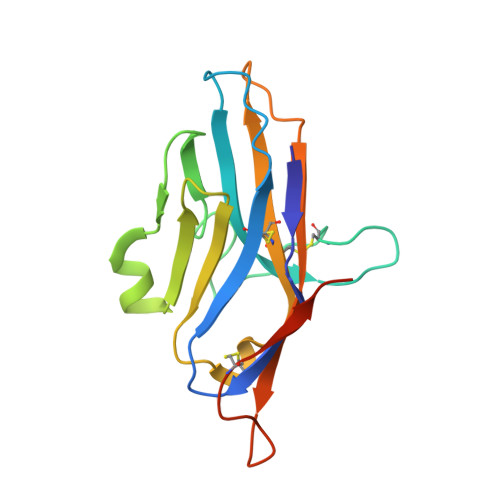
VISTA checkpoint inhibition by pH-selective antibody SNS-101 with optimized safety and pharmacokinetic profiles enhances PD-1 response.
Thisted, T., Smith, F.D., Mukherjee, A., Kleschenko, Y., Feng, F., Jiang, Z.G., Eitas, T., Malhotra, K., Biesova, Z., Onumajuru, A., Finley, F., Cifuentes, A., Zhang, G., Martin, G.H., Takeuchi, Y., Thiam, K., Schreiber, R.D., van der Horst, E.H.(2024) Nat Commun 15: 2917-2917
- PubMed: 38575562
- DOI: https://doi.org/10.1038/s41467-024-47256-x
- Primary Citation of Related Structures:
8TBQ - PubMed Abstract:
VISTA, an inhibitory myeloid-T-cell checkpoint, holds promise as a target for cancer immunotherapy. However, its effective targeting has been impeded by issues such as rapid clearance and cytokine release syndrome observed with previous VISTA antibodies. Here we demonstrate that SNS-101, a newly developed pH-selective VISTA antibody, addresses these challenges. Structural and biochemical analyses confirmed the pH-selectivity and unique epitope targeted by SNS-101. These properties confer favorable pharmacokinetic and safety profiles on SNS-101. In syngeneic tumor models utilizing human VISTA knock-in mice, SNS-101 shows in vivo efficacy when combined with a PD-1 inhibitor, modulates cytokine and chemokine signaling, and alters the tumor microenvironment. In summary, SNS-101, currently in Phase I clinical trials, emerges as a promising therapeutic biologic for a wide range of patients whose cancer is refractory to current immunotherapy regimens.
- Sensei Biotherapeutics Inc., 1405 Research Blvd, Suite 125, Rockville, MD, 20850, USA.
Organizational Affiliation: