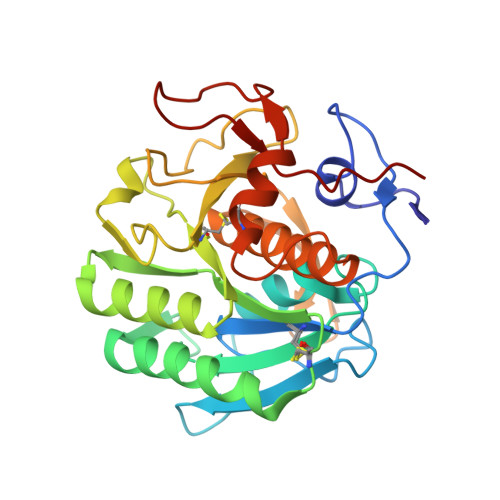
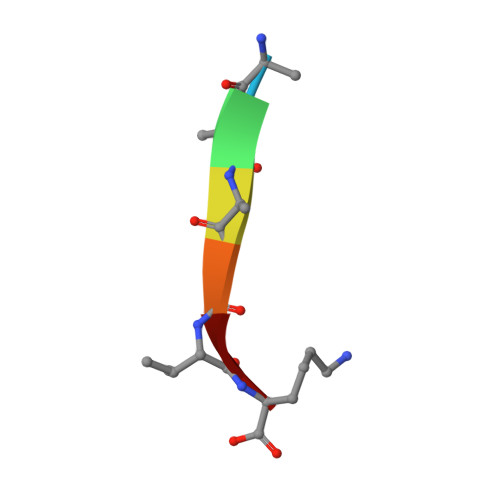
Refinement of multiconformer ensemble models from multi-temperature X-ray diffraction data.
Du, S., Wankowicz, S.A., Yabukarski, F., Doukov, T., Herschlag, D., Fraser, J.S.(2023) Methods Enzymol 688: 223-254
- PubMed: 37748828
- DOI: https://doi.org/10.1016/bs.mie.2023.06.009
- Primary Citation of Related Structures:
8SOG, 8SOU, 8SOV, 8SPL, 8SQV - PubMed Abstract:
Conformational ensembles underlie all protein functions. Thus, acquiring atomic-level ensemble models that accurately represent conformational heterogeneity is vital to deepen our understanding of how proteins work. Modeling ensemble information from X-ray diffraction data has been challenging, as traditional cryo-crystallography restricts conformational variability while minimizing radiation damage. Recent advances have enabled the collection of high quality diffraction data at ambient temperatures, revealing innate conformational heterogeneity and temperature-driven changes. Here, we used diffraction datasets for Proteinase K collected at temperatures ranging from 313 to 363 K to provide a tutorial for the refinement of multiconformer ensemble models. Integrating automated sampling and refinement tools with manual adjustments, we obtained multiconformer models that describe alternative backbone and sidechain conformations, their relative occupancies, and interconnections between conformers. Our models revealed extensive and diverse conformational changes across temperature, including increased bound peptide ligand occupancies, different Ca 2+ binding site configurations and altered rotameric distributions. These insights emphasize the value and need for multiconformer model refinement to extract ensemble information from diffraction data and to understand ensemble-function relationships.
- Department of Biochemistry, Stanford University, Stanford, CA, United States; Department of Chemistry, Stanford University, Stanford, CA, United States.
Organizational Affiliation: