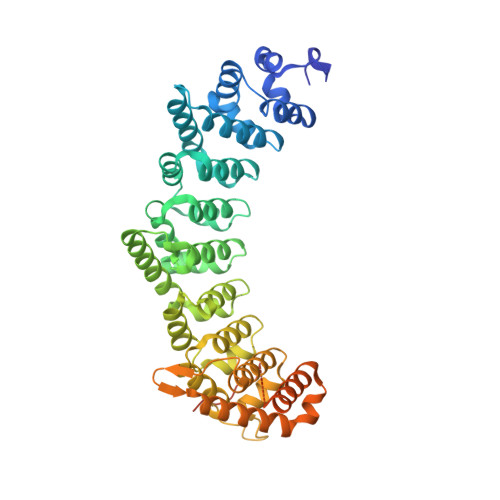
Intra- and inter-molecular regulation by intrinsically-disordered regions governs PUF protein RNA binding.
Qiu, C., Zhang, Z., Wine, R.N., Campbell, Z.T., Zhang, J., Hall, T.M.T.(2023) Nat Commun 14: 7323-7323
- PubMed: 37953271
- DOI: https://doi.org/10.1038/s41467-023-43098-1
- Primary Citation of Related Structures:
8SJ7 - PubMed Abstract:
PUF proteins are characterized by globular RNA-binding domains. They also interact with partner proteins that modulate their RNA-binding activities. Caenorhabditis elegans PUF protein fem-3 binding factor-2 (FBF-2) partners with intrinsically disordered Lateral Signaling Target-1 (LST-1) to regulate target mRNAs in germline stem cells. Here, we report that an intrinsically disordered region (IDR) at the C-terminus of FBF-2 autoinhibits its RNA-binding affinity by increasing the off rate for RNA binding. Moreover, the FBF-2 C-terminal region interacts with its globular RNA-binding domain at the same site where LST-1 binds. This intramolecular interaction restrains an electronegative cluster of amino acid residues near the 5' end of the bound RNA to inhibit RNA binding. LST-1 binding in place of the FBF-2 C-terminus therefore releases autoinhibition and increases RNA-binding affinity. This regulatory mechanism, driven by IDRs, provides a biochemical and biophysical explanation for the interdependence of FBF-2 and LST-1 in germline stem cell self-renewal.
- Epigenetics and Stem Cell Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA.
Organizational Affiliation: