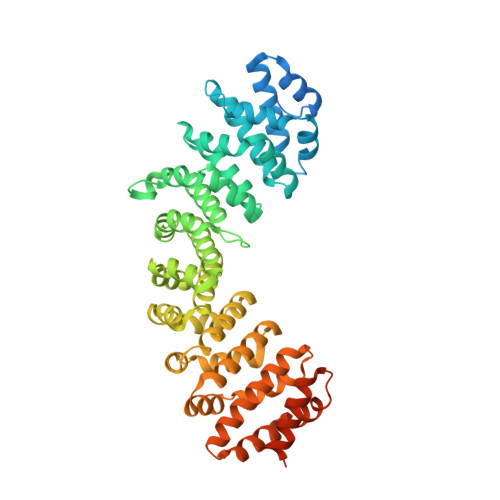
Structural basis for nuclear import of bat adeno-associated virus capsid protein.
Hoad, M., Roby, J.A., Forwood, J.K.(2024) J Gen Virol 105
- PubMed: 38441555
- DOI: https://doi.org/10.1099/jgv.0.001960
- Primary Citation of Related Structures:
8SG7 - PubMed Abstract:
Adeno-associated viruses (AAV) are one of the world's most promising gene therapy vectors and as a result, are one of the most intensively studied viral vectors. Despite a wealth of research into these vectors, the precise characterisation of AAVs to translocate into the host cell nucleus remains unclear. Recently we identified the nuclear localization signals of an AAV porcine strain and determined its mechanism of binding to host importin proteins. To expand our understanding of diverse AAV import mechanisms we sought to determine the mechanism in which the Cap protein from a bat-infecting AAV can interact with transport receptor importins for translocation into the nucleus. Using a high-resolution crystal structure and quantitative assays, we were able to not only determine the exact region and residues of the N-terminal domain of the Cap protein which constitute the functional NLS for binding with the importin alpha two protein, but also reveal the differences in binding affinity across the importin-alpha isoforms. Collectively our results allow for a detailed molecular view of the way AAV Cap proteins interact with host proteins for localization into the cell nucleus.
- School of Dentistry and Medical Sciences, Charles Sturt University, Wagga Wagga, NSW, 2678, Australia.
Organizational Affiliation: