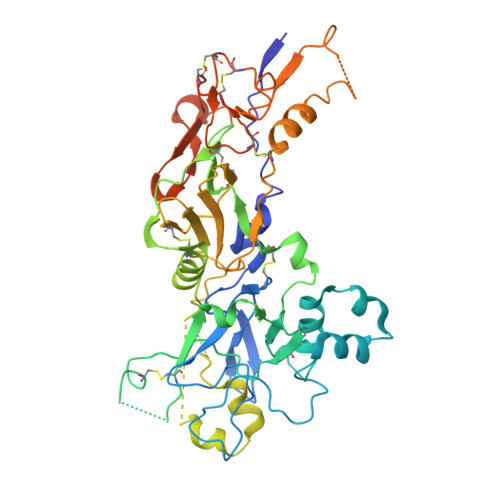
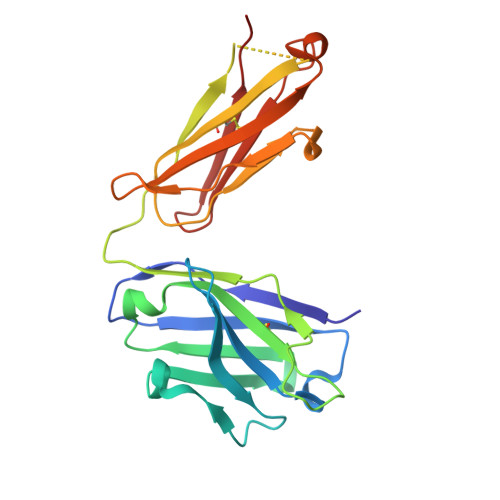
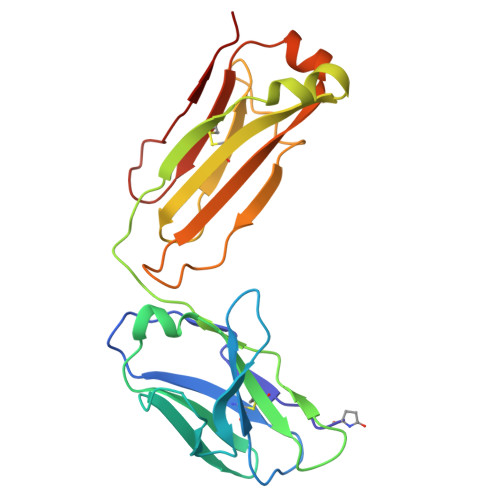
Conformational variability in the D2 loop of Plasmodium Apical Membrane antigen 1.
Saul, F.A., Vulliez-Le Normand, B., Boes, A., Spiegel, H., Kocken, C.H.M., Faber, B.W., Bentley, G.A.(2024) J Struct Biol X 10: 100110-100110
- PubMed: 39324028
- DOI: https://doi.org/10.1016/j.yjsbx.2024.100110
- Primary Citation of Related Structures:
8REK, 8REL, 9EVN, 9EVO - PubMed Abstract:
Apical Membrane Antigen 1 (AMA1) plays a vital role in the invasion of the host erythrocyte by the malaria parasite, Plasmodium . It is thus an important target for vaccine and anti-malaria therapeutic strategies that block the invasion process. AMA1, present on the surface of the parasite, interacts with RON2, a component of the parasite's rhoptry neck (RON) protein complex, which is transferred to the erythrocyte membrane during invasion. The D2 loop of AMA1 plays an essential role in invasion as it partially covers the RON2-binding site and must therefore be displaced for invasion to proceed. Several structural studies have shown that the D2 loop is very mobile, a property that is probably important for the function of AMA1. Here we present three crystal structures of AMA1 from P. falciparum (strains 3D7 and FVO) and P. vivax (strain Sal1), in which the D2 loop could be largely traced in the electron density maps. The D2 loop of PfAMA1-FVO and PvAMA1 (as a complex with a monoclonal antibody Fab) has a conformation previously noted in the P. knowlesi AMA1 structure. The D2 loop of PfAMA1-3D7, however, reveals a novel conformation. We analyse the conformational variability of the D2 loop in these structures, together with those previously reported. Three different conformations can be distinguished, all of which are highly helical and show some similarity in their secondary structure organisation. We discuss the significance of these observations in the light of the flexible nature of the D2 loop and its role in AMA1 function.
- Institut Pasteur, Université Paris Cité, CNRS UMR 3528, Plate-forme de Cristallographie C2RT, 75015 Paris, France.
Organizational Affiliation: