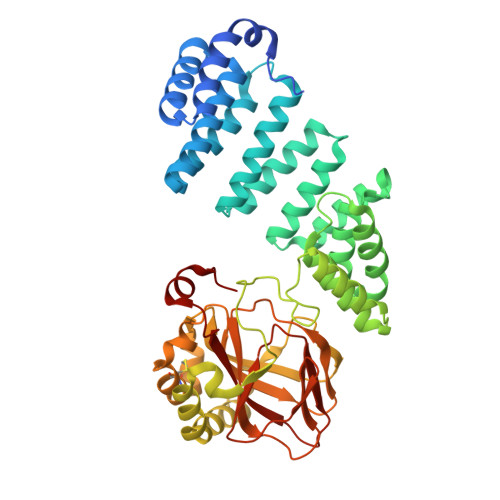
Structural and functional consequences of aspartate/asparagine-beta-hydroxylase variants causing Traboulsi Syndrome.
Hou, C.X., Brasnett, A., Rabe, P., Schofield, C.J., Brewitz, L.(2025) J Biological Chem : 111008-111008
- PubMed: 41354343
- DOI: https://doi.org/10.1016/j.jbc.2025.111008
- Primary Citation of Related Structures:
8RE5, 8RE6, 8RE7, 8RE8, 8RE9 - PubMed Abstract:
Traboulsi Syndrome is an autosomal recessive hereditary disease associated with developmental defects, in particular of the ocular system. Single nucleotide polymorphisms affecting the ASPH gene, which encodes for the 2-oxoglutarate (2OG)-dependent oxygenase aspartate/asparagine-β-hydroxylase (AspH), are associated with Traboulsi Syndrome. AspH catalyzes hydroxylations of conserved aspartate/asparagine residues in epidermal growth factor-like domain (EGFD) proteins. We report studies on the clinically-observed Traboulsi Syndrome-associated R688Q, R735Q, and R735W AspH variants. The results reveal that pathogenic active site substitutions substantially reduce, though do not ablate, EGFD hydroxylase activity compared to wildtype AspH. They imply that efficient AspH catalyzed EGFD hydroxylation is important during human development. Crystallographic studies reveal conservation of the overall AspH fold, but that the preferred conformations of 2OG in complex with the R735Q and R735W AspH variants differ from that with wildtype AspH. Screening of potential 2OG cosubstrate substitutes reveals certain 2-oxoacids, including naturally present metabolites, manifest enhanced catalytic efficiency of Traboulsi Syndrome-associated AspH variants compared to 2OG. The results thus provide proof-of-principle for a therapeutic strategy involving rescue of impaired activities of pathogenic active site AspH variants by use of 2-oxoacids, or 2-oxoacid precursors, other than 2OG.
- Chemistry Research Laboratory and the Ineos Oxford Institute for Antimicrobial Research, University of Oxford, 12 Mansfield Road, OX1 3TA, Oxford, United Kingdom.
Organizational Affiliation: