Crystal structure of ERK2 in complex with a covalently bound macrocyclic ligand
Gelin, M., Labesse, G., Guichou, J.-F., Pons, J.-L., Barluenga, S., Katickeyan, G., Winssinger, N.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Mitogen-activated protein kinase 1 | 364 | Rattus norvegicus | Mutation(s): 0 Gene Names: Mapk1 EC: 2.7.11.24 | 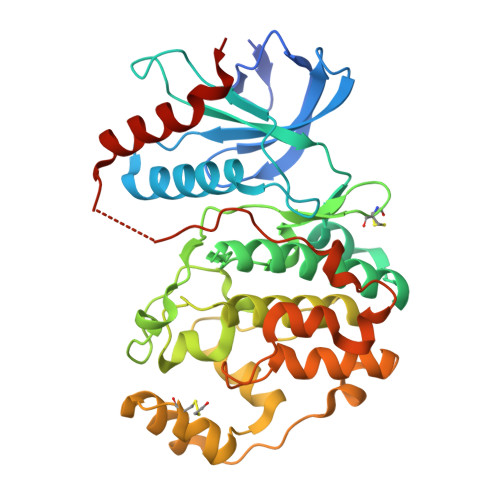 | |
UniProt | |||||
Find proteins for P63086 (Rattus norvegicus) Explore P63086 Go to UniProtKB: P63086 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P63086 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| WM6 (Subject of Investigation/LOI) Query on WM6 | D [auth A] | (4~{R},5~{S},6~{S})-4,5,6,15,17-pentakis(oxidanyl)-2,12-dioxabicyclo[12.4.0]octadeca-1(14),15,17-triene-7,13-dione C16 H20 O9 JTDNYLVWHABXBF-UGFHNGPFSA-N |  | ||
| SO4 Query on SO4 | B [auth A], C [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CME Query on CME | A | L-PEPTIDE LINKING | C5 H11 N O3 S2 |  | CYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 48.65 | α = 90 |
| b = 69.84 | β = 109.38 |
| c = 59.77 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| Aimless | data scaling |
| MOSFLM | data reduction |
| PHENIX | phasing |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Agence Nationale de la Recherche (ANR) | France | ANR-20-CE18-0025 |