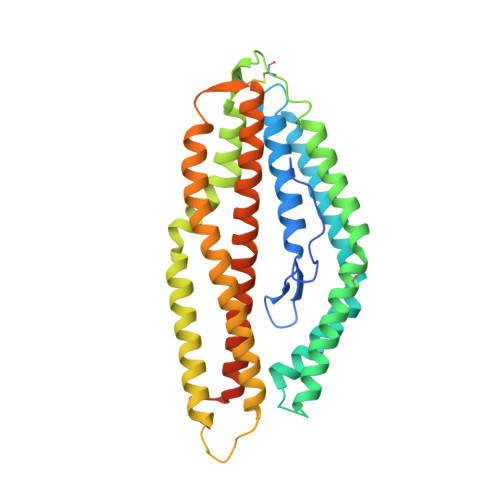
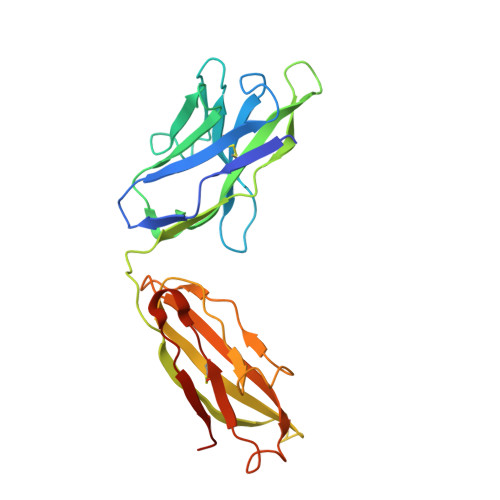
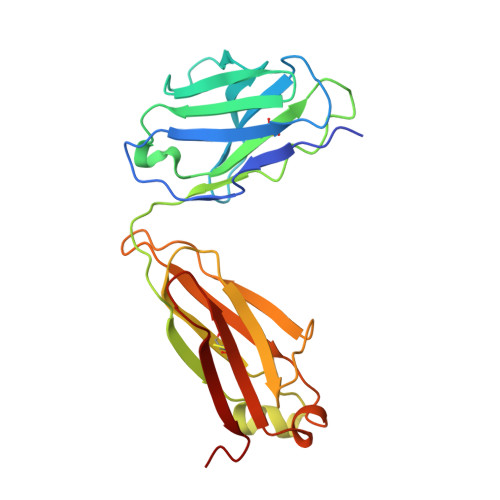
Analysis of the diverse antigenic landscape of the malaria protein RH5 identifies a potent vaccine-induced human public antibody clonotype.
Barrett, J.R., Pipini, D., Wright, N.D., Cooper, A.J.R., Gorini, G., Quinkert, D., Lias, A.M., Davies, H., Rigby, C.A., Aleshnick, M., Williams, B.G., Bradshaw, W.J., Paterson, N.G., Martinson, T., Kirtley, P., Picard, L., Wiggins, C.D., Donnellan, F.R., King, L.D.W., Wang, L.T., Popplewell, J.F., Silk, S.E., de Ruiter Swain, J., Skinner, K., Kotraiah, V., Noe, A.R., MacGill, R.S., King, C.R., Birkett, A.J., Soisson, L.A., Minassian, A.M., Lauffenburger, D.A., Miura, K., Long, C.A., Wilder, B.K., Koekemoer, L., Tan, J., Nielsen, C.M., McHugh, K., Draper, S.J.(2024) Cell 187: 4964
- PubMed: 39059380
- DOI: https://doi.org/10.1016/j.cell.2024.06.015
- Primary Citation of Related Structures:
8QKR, 8QKS - PubMed Abstract:
The highly conserved and essential Plasmodium falciparum reticulocyte-binding protein homolog 5 (PfRH5) has emerged as the leading target for vaccines against the disease-causing blood stage of malaria. However, the features of the human vaccine-induced antibody response that confer highly potent inhibition of malaria parasite invasion into red blood cells are not well defined. Here, we characterize 236 human IgG monoclonal antibodies, derived from 15 donors, induced by the most advanced PfRH5 vaccine. We define the antigenic landscape of this molecule and establish that epitope specificity, antibody association rate, and intra-PfRH5 antibody interactions are key determinants of functional anti-parasitic potency. In addition, we identify a germline IgG gene combination that results in an exceptionally potent class of antibody and demonstrate its prophylactic potential to protect against P. falciparum parasite challenge in vivo. This comprehensive dataset provides a framework to guide rational design of next-generation vaccines and prophylactic antibodies to protect against blood-stage malaria.
- Department of Biochemistry, University of Oxford, Oxford OX1 3QU, UK; Kavli Institute for Nanoscience Discovery, University of Oxford, Oxford OX1 3QU, UK; The Jenner Institute, University of Oxford, Oxford OX3 7DQ, UK.
Organizational Affiliation: