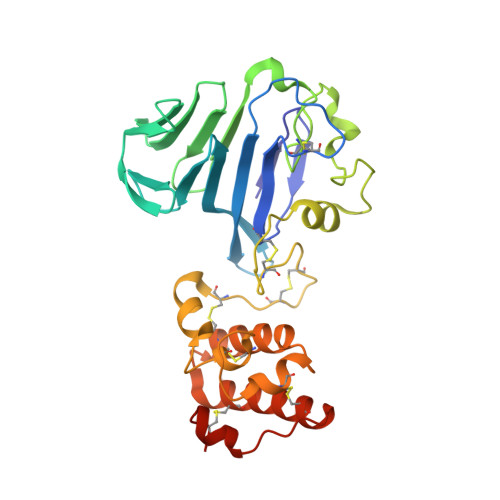
VWD domain stabilization by autocatalytic Asp-Pro cleavage.
Yeshaya, N., Gupta, P.K., Dym, O., Morgenstern, D., Major, D.T., Fass, D.(2024) Protein Sci 33: e4929-e4929
- PubMed: 38380729
- DOI: https://doi.org/10.1002/pro.4929
- Primary Citation of Related Structures:
8QCI - PubMed Abstract:
Domains known as von Willebrand factor type D (VWD) are found in extracellular and cell-surface proteins including von Willebrand factor, mucins, and various signaling molecules and receptors. Many VWD domains have a glycine-aspartate-proline-histidine (GDPH) amino-acid sequence motif, which is hydrolytically cleaved post-translationally between the aspartate (Asp) and proline (Pro). The Fc IgG binding protein (FCGBP), found in intestinal mucus secretions and other extracellular environments, contains 13 VWD domains, 11 of which have a GDPH cleavage site. In this study, we investigated the structural and biophysical consequences of Asp-Pro peptide cleavage in a representative FCGBP VWD domain. We found that endogenous Asp-Pro cleavage increases the resistance of the domain to exogenous proteolytic degradation. Tertiary structural interactions made by the newly generated chain termini, as revealed by a crystal structure of an FCGBP segment containing the VWD domain, may explain this observation. Notably, the Gly-Asp peptide bond, upstream of the cleavage site, assumed the cis configuration in the structure. In addition to these local features of the cleavage site, a global organizational difference was seen when comparing the FCGBP segment structure with the numerous other structures containing the same set of domains. Together, these data illuminate the outcome of GDPH cleavage and demonstrate the plasticity of proteins with VWD domains, which may contribute to their evolution for function in a dynamic extracellular environment.
- Department of Chemical and Structural Biology, Weizmann Institute of Science, Rehovot, Israel.
Organizational Affiliation: