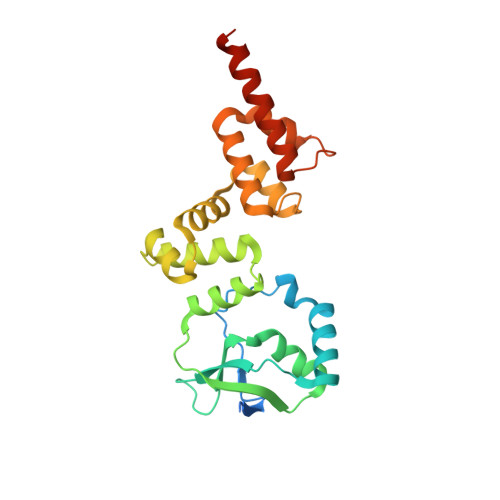
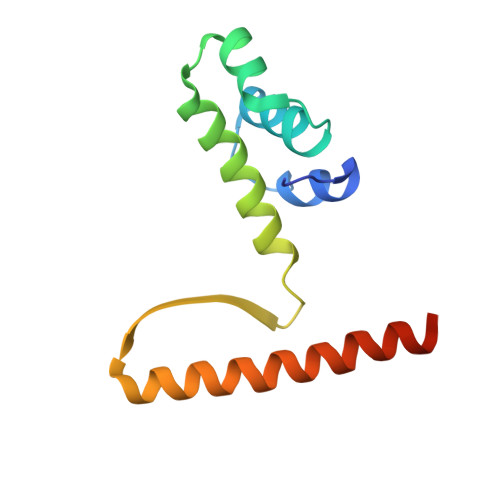
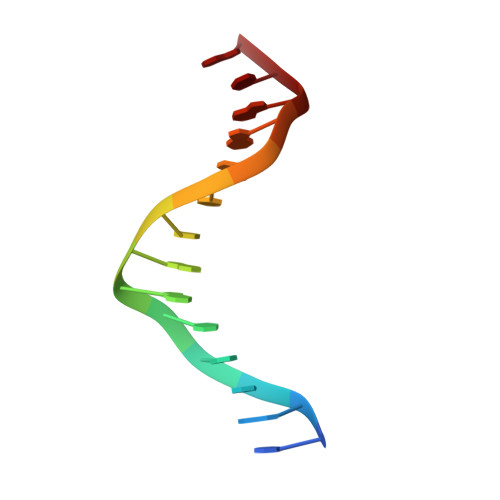
KorB switching from DNA-sliding clamp to repressor mediates long-range gene silencing in a multi-drug resistance plasmid.
McLean, T.C., Balaguer-Perez, F., Chandanani, J., Thomas, C.M., Aicart-Ramos, C., Burick, S., Olinares, P.D.B., Gobbato, G., Mundy, J.E.A., Chait, B.T., Lawson, D.M., Darst, S.A., Campbell, E.A., Moreno-Herrero, F., Le, T.B.K.(2025) Nat Microbiol 10: 448-467
- PubMed: 39849085
- DOI: https://doi.org/10.1038/s41564-024-01915-3
- Primary Citation of Related Structures:
8QA8, 8QA9 - PubMed Abstract:
Examples of long-range gene regulation in bacteria are rare and generally thought to involve DNA looping. Here, using a combination of biophysical approaches including X-ray crystallography and single-molecule analysis for the KorB-KorA system in Escherichia coli, we show that long-range gene silencing on the plasmid RK2, a source of multi-drug resistance across diverse Gram-negative bacteria, is achieved cooperatively by a DNA-sliding clamp, KorB, and a clamp-locking protein, KorA. We show that KorB is a CTPase clamp that can entrap and slide along DNA to reach distal target promoters up to 1.5 kb away. We resolved the tripartite crystal structure of a KorB-KorA-DNA co-complex, revealing that KorA latches KorB into a closed clamp state. DNA-bound KorA thus stimulates repression by stalling KorB sliding at target promoters to occlude RNA polymerase holoenzymes. Together, our findings explain the mechanistic basis for KorB role switching from a DNA-sliding clamp to a co-repressor and provide an alternative mechanism for long-range regulation of gene expression in bacteria.
- Department of Molecular Microbiology, John Innes Centre, Norwich, UK. thomas.mcLean@jic.ac.uk.
Organizational Affiliation: