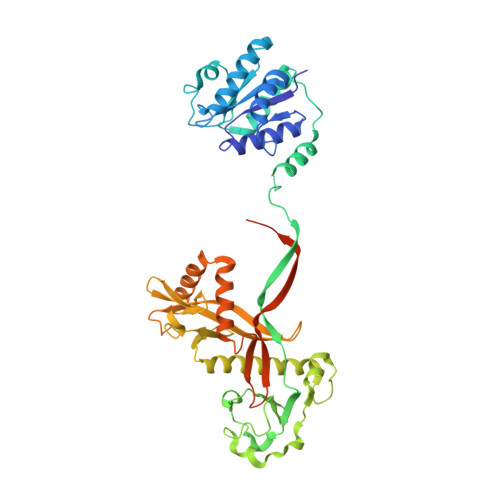
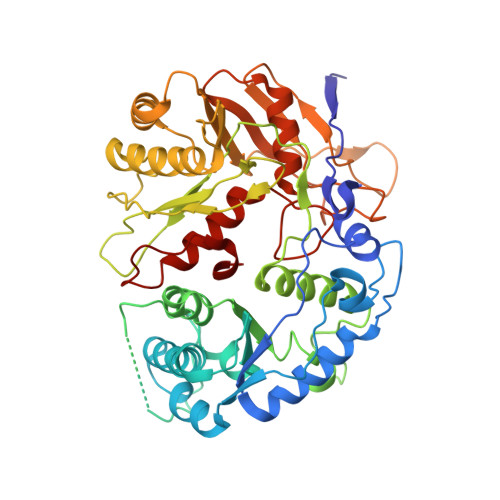
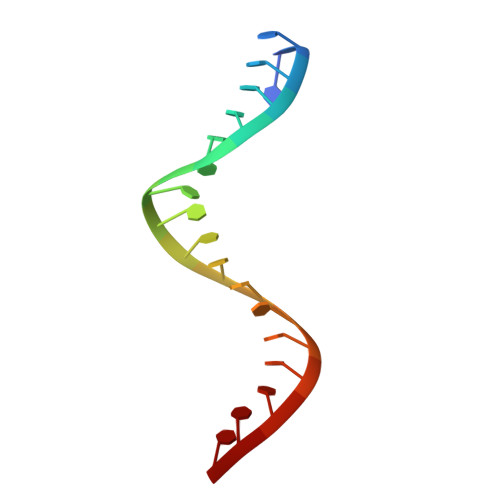
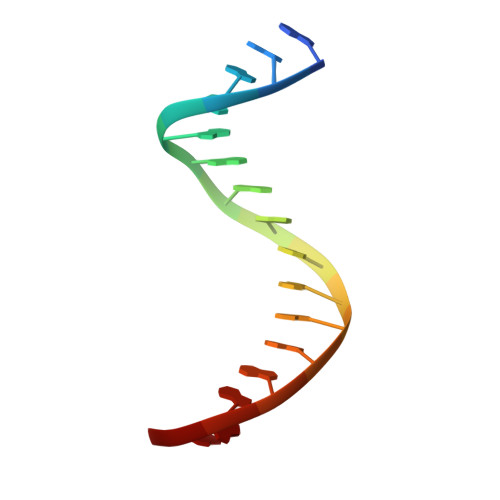
Activation mechanism of a short argonaute-TIR prokaryotic immune system.
Ni, D., Lu, X., Stahlberg, H., Ekundayo, B.(2023) Sci Adv 9: eadh9002-eadh9002
- PubMed: 37467330
- DOI: https://doi.org/10.1126/sciadv.adh9002
- Primary Citation of Related Structures:
8OZ6, 8OZC, 8OZD, 8OZE, 8OZF, 8OZG, 8OZI - PubMed Abstract:
Short prokaryotic argonaute (pAgo) and toll/interleukin-1 receptor/resistance protein (TIR)-analog of PAZ (APAZ) form a heterodimeric SPARTA complex that provides immunity to its prokaryotic host through an abortive infection mechanism. Monomeric SPARTA senses foreign RNA/DNA duplexes to assemble an active tetramer resulting in cell death by nicotinamide adenine dinucleotide (oxidized form) (NAD) depletion via an unknown mechanism. We report nine structures of SPARTA in different functional states at a resolution range of 4.2 to 2.9 angstroms, revealing its activation mechanism. Inactive SPARTA monomers bind to RNA/DNA duplexes to form symmetric dimers mediated by the association of Ago subunits. The initiation of tetramer assembly induces flexibility of the TIR domains enabling a symmetry-breaking rotational movement of a TIR domain in the dimer units which facilitates the TIR oligomerization, resulting in the formation of the substrate binding pocket and the activation of the SPARTA complex's NADase activity. Our findings provide detailed structural and mechanistic insights into activating a short argonaute defense system.
- Laboratory of Biological Electron Microscopy, IPHYS, SB, EPFL, and Dept. Fundamental Microbiology, Faculty of Biology and Medicine, UNIL, Cubotron, Rt. de la Sorge, 1015 Lausanne, Switzerland.
Organizational Affiliation: