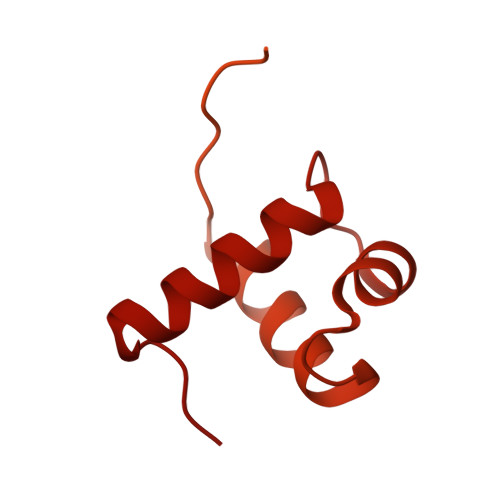
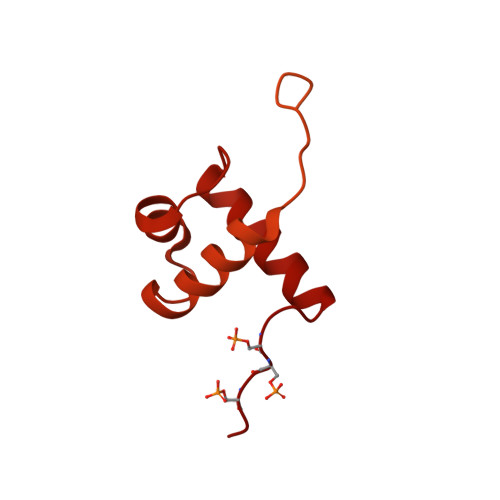
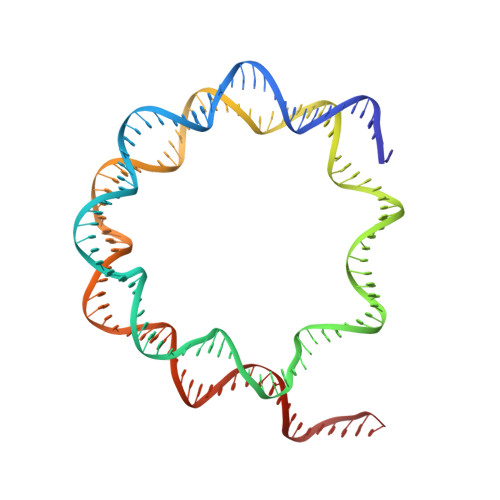
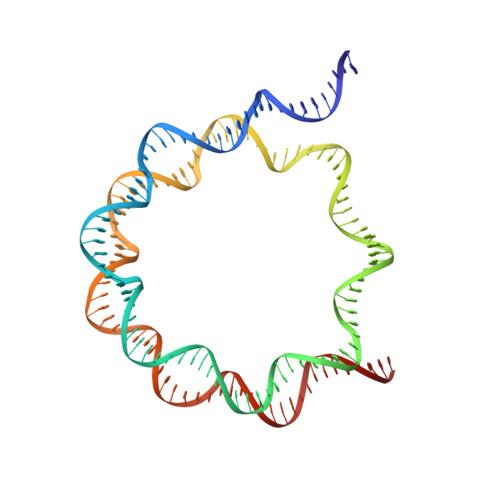
Structural basis of telomeric nucleosome recognition by shelterin factor TRF1.
Hu, H., van Roon, A.M., Ghanim, G.E., Ahsan, B., Oluwole, A.O., Peak-Chew, S.Y., Robinson, C.V., Nguyen, T.H.D.(2023) Sci Adv 9: eadi4148-eadi4148
- PubMed: 37624885
- DOI: https://doi.org/10.1126/sciadv.adi4148
- Primary Citation of Related Structures:
8OX0, 8OX1 - PubMed Abstract:
Shelterin and nucleosomes are the key players that organize mammalian chromosome ends into the protective telomere caps. However, how they interact with each other at telomeres remains unknown. We report cryo-electron microscopy structures of a human telomeric nucleosome both unbound and bound to the shelterin factor TRF1. Our structures reveal that TRF1 binds unwrapped nucleosomal DNA ends by engaging both the nucleosomal DNA and the histone octamer. Unexpectedly, TRF1 binding shifts the register of the nucleosomal DNA by 1 bp. We discovered that phosphorylation of the TRF1 C terminus and a noncanomical DNA binding surface on TRF1 are critical for its association with telomeric nucleosomes. These insights into shelterin-chromatin interactions have crucial implications for understanding telomeric chromatin organization and other roles of shelterin at telomeres including replication and transcription.
- MRC Laboratory of Molecular Biology, Cambridge, CB2 0QH, UK.
Organizational Affiliation: