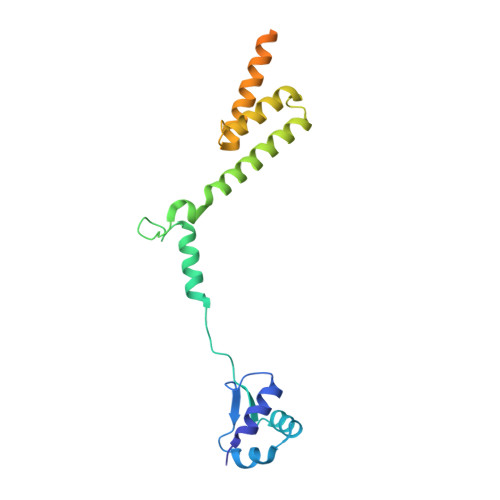
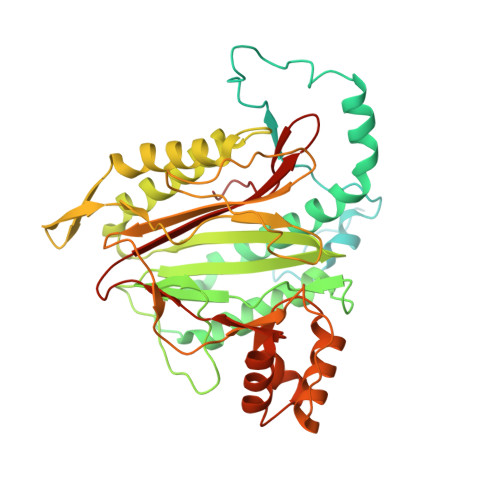
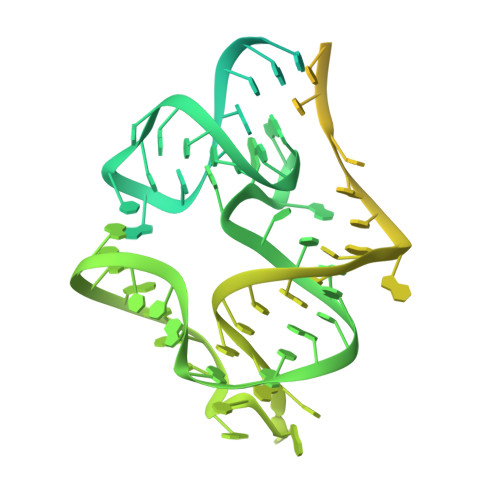
Methionine aminopeptidase 2 and its autoproteolysis product have different binding sites on the ribosome.
Klein, M.A., Wild, K., Kisonaite, M., Sinning, I.(2024) Nat Commun 15: 716-716
- PubMed: 38267453
- DOI: https://doi.org/10.1038/s41467-024-44862-7
- Primary Citation of Related Structures:
8ONX, 8ONY, 8ONZ, 8OO0 - PubMed Abstract:
Excision of the initiator methionine is among the first co-translational processes that occur at the ribosome. While this crucial step in protein maturation is executed by two types of methionine aminopeptidases in eukaryotes (MAP1 and MAP2), additional roles in disease and translational regulation have drawn more attention to MAP2. Here, we report several cryo-EM structures of human and fungal MAP2 at the 80S ribosome. Irrespective of nascent chains, MAP2 can occupy the tunnel exit. On nascent chain displaying ribosomes, the MAP2-80S interaction is highly dynamic and the MAP2-specific N-terminal extension engages in stabilizing interactions with the long rRNA expansion segment ES27L. Loss of this extension by autoproteolytic cleavage impedes interactions at the tunnel, while promoting MAP2 to enter the ribosomal A-site, where it engages with crucial functional centers of translation. These findings reveal that proteolytic remodeling of MAP2 severely affects ribosome binding, and set the stage for targeted functional studies.
- Heidelberg University Biochemistry Center (BZH), Im Neuenheimer Feld 328, 69120, Heidelberg, Germany.
Organizational Affiliation: