Structure basis of ligand selectivity and signal transduction in human melanocortin-3 receptor
Jingpeng, F., Hongli, H.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Soluble cytochrome b562,Melanocortin receptor 3,GlgA glycogen synthase,Fusion protein | A [auth R] | 619 | Escherichia coli, Homo sapiens, Pyrococcus abyssi GE5, synthetic construct This entity is chimeric | Mutation(s): 0 Gene Names: cybC, MC3R, PAB2292 Membrane Entity: Yes | 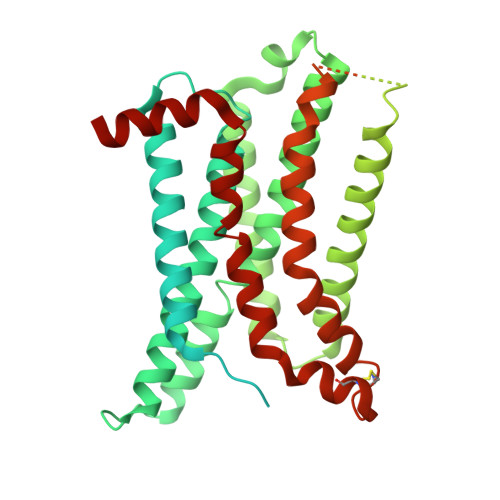 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P41968 (Homo sapiens) Explore P41968 Go to UniProtKB: P41968 | |||||
PHAROS: P41968 GTEx: ENSG00000124089 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P41968 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| SHU9119 | B [auth L] | 9 | synthetic construct | Mutation(s): 0 |  |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CA (Subject of Investigation/LOI) Query on CA | C [auth R] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| NLE Query on NLE | B [auth L] | L-PEPTIDE LINKING | C6 H13 N O2 |  | LEU |
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.20.1_4487: |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Other government | Shenzhen Science and Technology Innovation Committee | |
| Other private | Ganghong Young Scholar Development Fund |