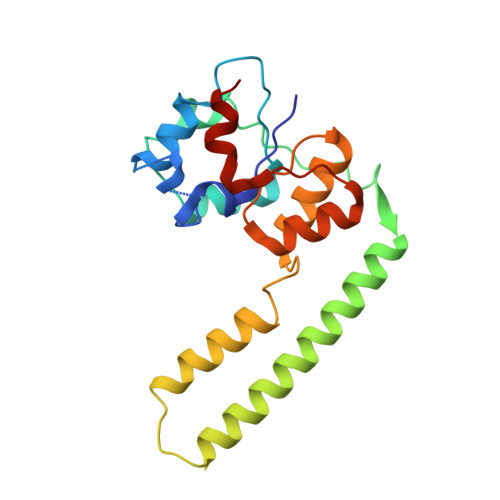
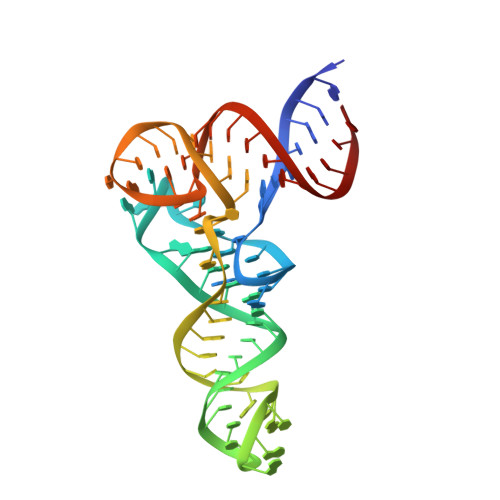
Structural basis of transfer RNA processing by bacterial minimal RNase P.
Teramoto, T., Koyasu, T., Yokogawa, T., Adachi, N., Mayanagi, K., Nakamura, T., Senda, T., Kakuta, Y.(2025) Nat Commun 16: 5456-5456
- PubMed: 40593470
- DOI: https://doi.org/10.1038/s41467-025-60002-1
- Primary Citation of Related Structures:
8KD9, 8KDA - PubMed Abstract:
Precursor tRNAs (pre-tRNAs) require nucleolytic removal of 5'-leader and 3'-trailer sequences for maturation, which is essential for proper tRNA function. The endoribonuclease RNase P exists in diverse forms, including RNA- and protein-based RNase P, and removes 5'-leader sequences from pre-tRNAs. Some bacteria and archaea possess a unique minimal protein-based RNase P enzyme, HARP, which forms dodecamers with twelve active sites. Here, we present cryogenic electron microscopy structures of HARP dodecamers complexed with five pre-tRNAs, and we show that HARP oligomerization enables specific recognition of the invariant distance between the acceptor stem 5'-end and the TψC-loop, functioning as a molecular ruler-a feature representing convergent evolution among RNase P enzymes. The HARP dodecamer uses only five active sites for 5'-leader cleavage, while we identify a 3'-trailer cleavage activity in the remaining seven sites. This elucidation reveals how small proteins evolve through oligomerization to adapt a pivotal biological function (5'-leader processing) and acquire a novel function (3'-trailer processing).
- Laboratory of Biophysical Chemistry, Department of Bioscience and Biotechnology, Faculty of Agriculture, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka, 819-0395, Japan. teramotot@agr.kyushu-u.ac.jp.
Organizational Affiliation: