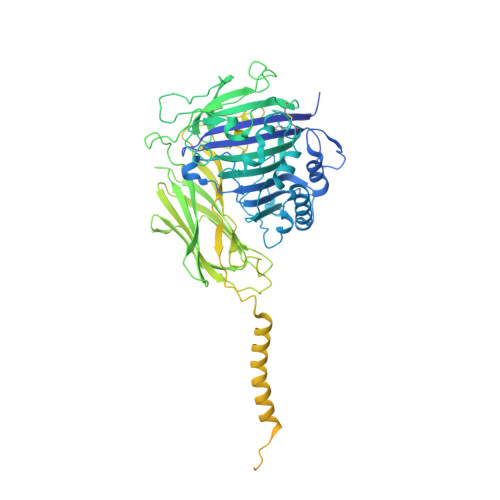
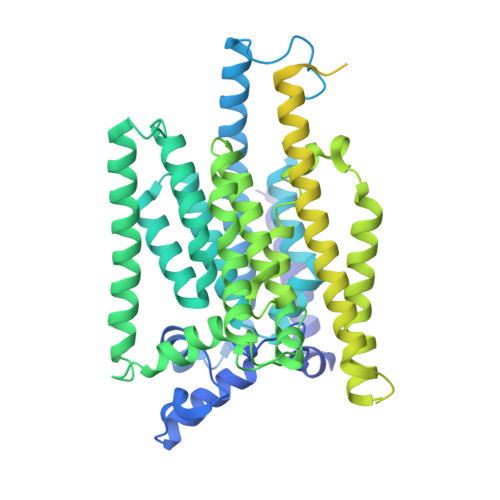
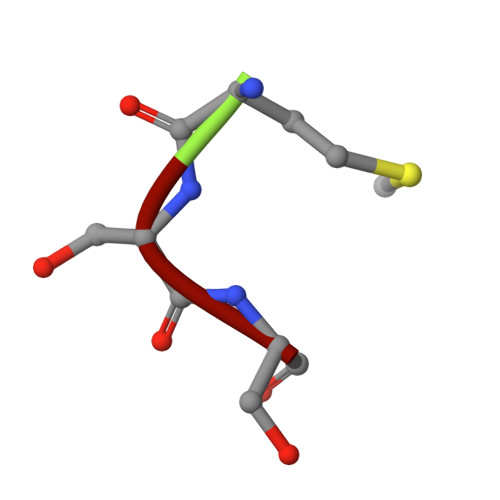
Structures of liganded glycosylphosphatidylinositol transamidase illuminate GPI-AP biogenesis.
Xu, Y., Li, T., Zhou, Z., Hong, J., Chao, Y., Zhu, Z., Zhang, Y., Qu, Q., Li, D.(2023) Nat Commun 14: 5520-5520
- PubMed: 37684232
- DOI: https://doi.org/10.1038/s41467-023-41281-y
- Primary Citation of Related Structures:
8IMX, 8IMY - PubMed Abstract:
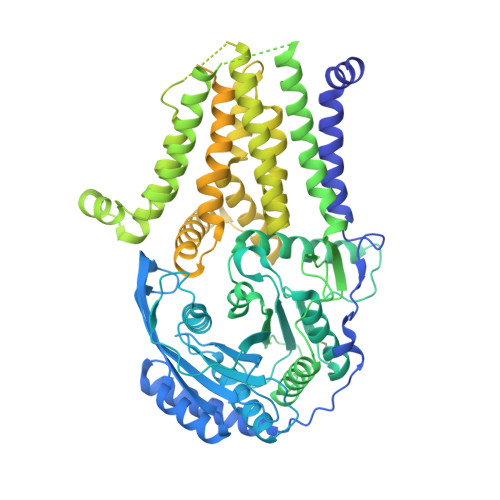
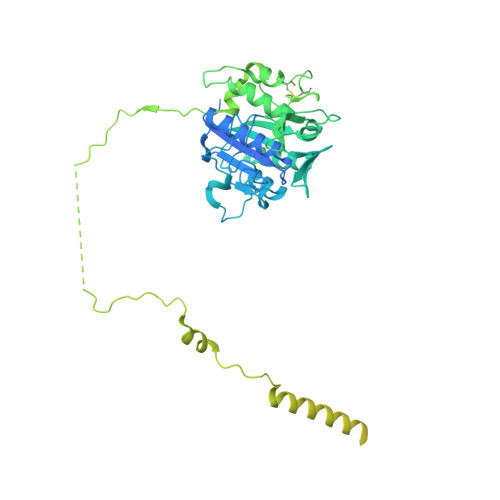
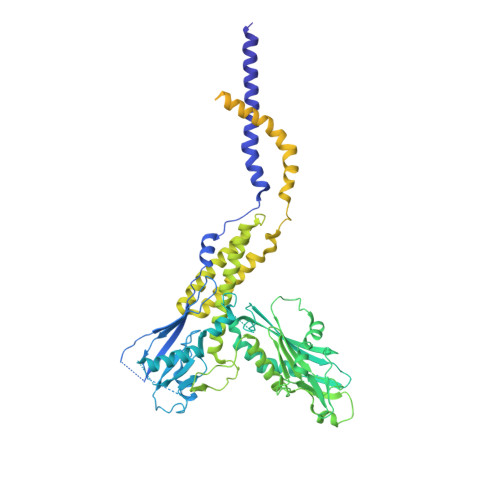
Many eukaryotic receptors and enzymes rely on glycosylphosphatidylinositol (GPI) anchors for membrane localization and function. The transmembrane complex GPI-T recognizes diverse proproteins at a signal peptide region that lacks consensus sequence and replaces it with GPI via a transamidation reaction. How GPI-T maintains broad specificity while preventing unintentional cleavage is unclear. Here, substrates- and products-bound human GPI-T structures identify subsite features that enable broad proprotein specificity, inform catalytic mechanism, and reveal a multilevel safeguard mechanism against its promiscuity. In the absence of proproteins, the catalytic site is invaded by a locally stabilized loop. Activation requires energetically unfavorable rearrangements that transform the autoinhibitory loop into crucial catalytic cleft elements. Enzyme-proprotein binding in the transmembrane and luminal domains respectively powers the conformational rearrangement and induces a competent cleft. GPI-T thus integrates various weak specificity regions to form strong selectivity and prevent accidental activation. These findings provide important mechanistic insights into GPI-anchored protein biogenesis.
- State Key Laboratory of Molecular Biology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (CAS), University of CAS, Shanghai, China.
Organizational Affiliation: