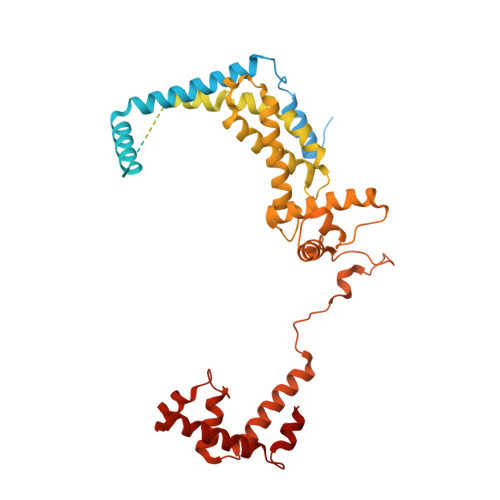
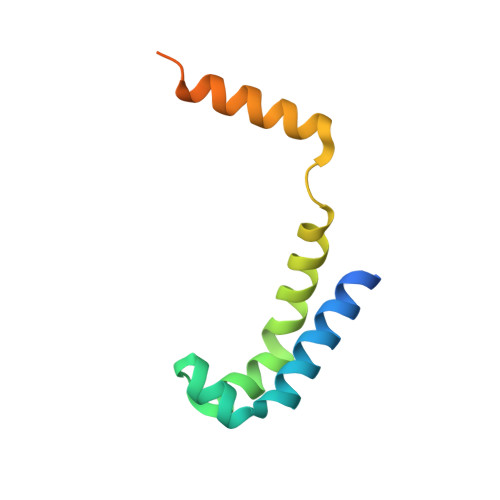
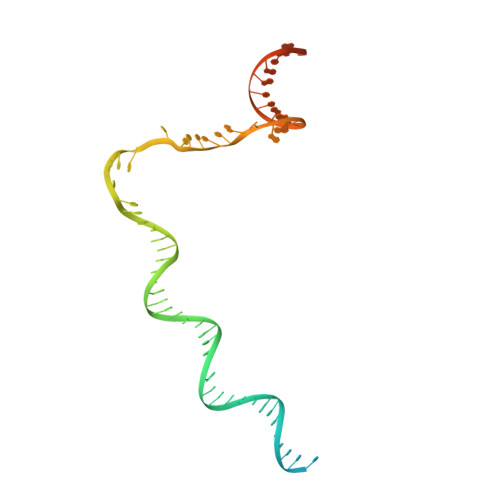
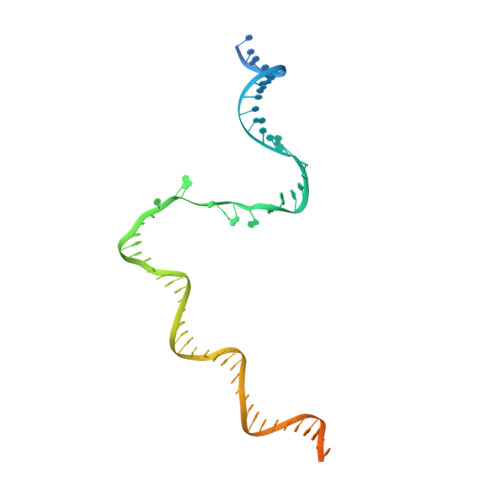
Structural basis of lambda CII-dependent transcription activation.
Zhao, M., Gao, B., Wen, A., Feng, Y., Lu, Y.Q.(2023) Structure 31: 968
- PubMed: 37269829
- DOI: https://doi.org/10.1016/j.str.2023.05.008
- Primary Citation of Related Structures:
8IGR, 8IGS - PubMed Abstract:
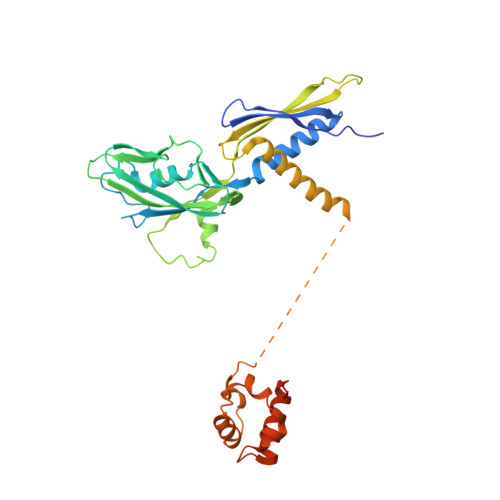
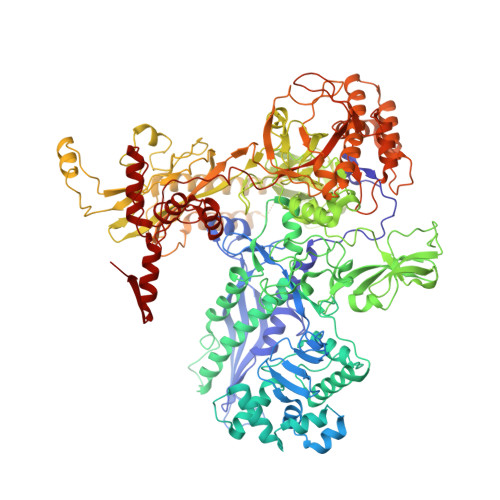
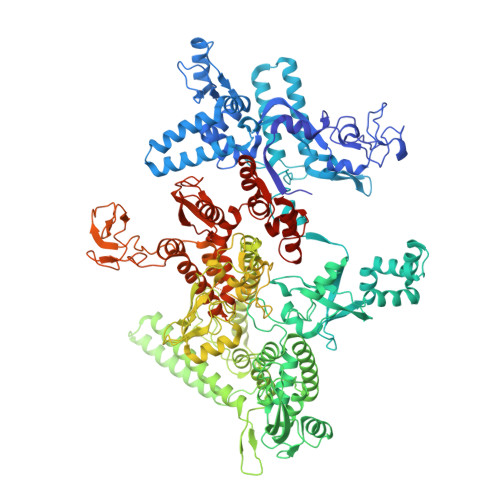
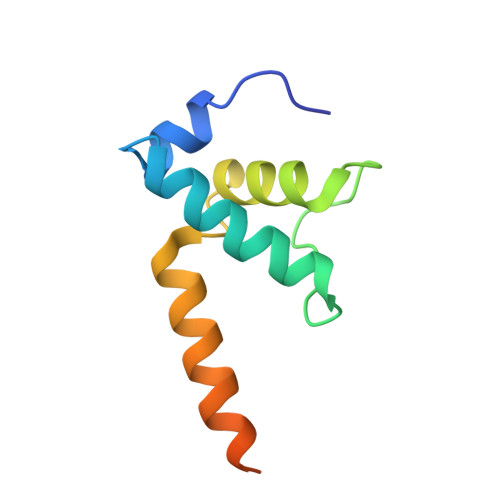
The CII protein of bacteriophage λ activates transcription from the phage promoters P RE , P I , and P AQ by binding to two direct repeats that straddle the promoter -35 element. Although genetic, biochemical, and structural studies have elucidated many aspects of λCII-mediated transcription activation, no precise structure of the transcription machinery in the process is available. Here, we report a 3.1-Å cryo-electron microscopy (cryo-EM) structure of an intact λCII-dependent transcription activation complex (TAC-λCII), which comprises λCII, E. coli RNAP-σ 70 holoenzyme, and the phage promoter P RE . The structure reveals the interactions between λCII and the direct repeats responsible for promoter specificity and the interactions between λCII and RNAP α subunit C-terminal domain responsible for transcription activation. We also determined a 3.4-Å cryo-EM structure of an RNAP-promoter open complex (RPo-P RE ) from the same dataset. Structural comparison between TAC-λCII and RPo-P RE provides new insights into λCII-dependent transcription activation.
- Department of Emergency Medicine of the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China.
Organizational Affiliation: