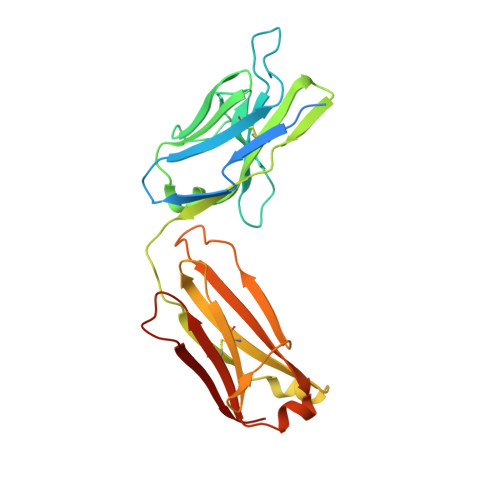
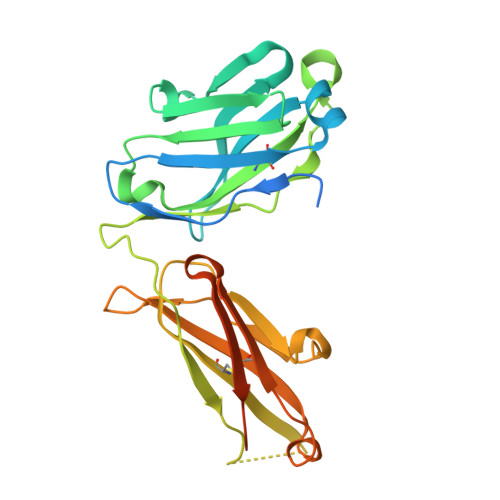
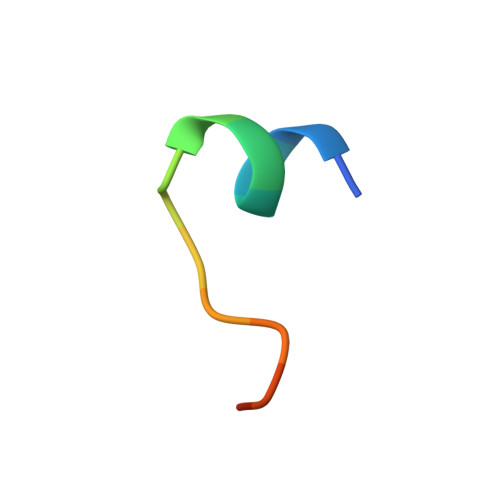
Structural basis for cross-group recognition of an influenza virus hemagglutinin antibody that targets postfusion stabilized epitope.
Tonouchi, K., Adachi, Y., Suzuki, T., Kuroda, D., Nishiyama, A., Yumoto, K., Takeyama, H., Suzuki, T., Hashiguchi, T., Takahashi, Y.(2023) PLoS Pathog 19: e1011554-e1011554
- PubMed: 37556494
- DOI: https://doi.org/10.1371/journal.ppat.1011554
- Primary Citation of Related Structures:
8IB1 - PubMed Abstract:
Plasticity of influenza virus hemagglutinin (HA) conformation increases an opportunity to generate conserved non-native epitopes with unknown functionality. Here, we have performed an in-depth analysis of human monoclonal antibodies against a stem-helix region that is occluded in native prefusion yet exposed in postfusion HA. A stem-helix antibody, LAH31, provided IgG Fc-dependent cross-group protection by targeting a stem-helix kinked loop epitope, with a unique structure emerging in the postfusion state. The structural analysis and molecular modeling revealed key contact sites responsible for the epitope specificity and cross-group breadth that relies on somatically mutated light chain. LAH31 was inaccessible to the native prefusion HA expressed on cell surface; however, it bound to the HA structure present on infected cells with functional linkage to the Fc-mediated clearance. Our study uncovers a novel non-native epitope that emerges in the postfusion HA state, highlighting the utility of this epitope for a broadly protective antigen design.
- Research Center for Drug and Vaccine Development, National Institute of Infectious Diseases, Shinjuku, Tokyo, Japan.
Organizational Affiliation: