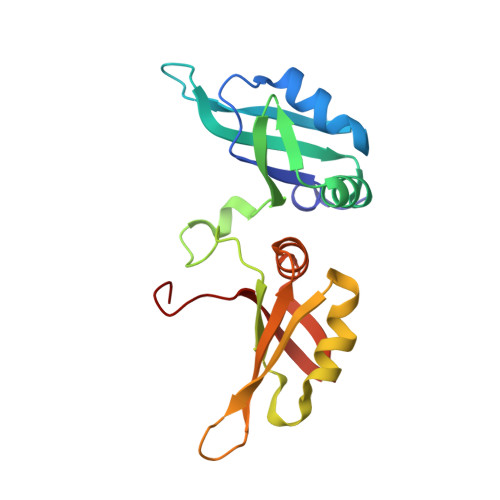
Structural Insight Into hnRNP A2/B1 Homodimerization and DNA Recognition.
Liu, Y., Abula, A., Xiao, H., Guo, H., Li, T., Zheng, L., Chen, B., Nguyen, H.C., Ji, X.(2023) J Mol Biology 435: 167920-167920
- PubMed: 36528084
- DOI: https://doi.org/10.1016/j.jmb.2022.167920
- Primary Citation of Related Structures:
7WM3, 8HNI - PubMed Abstract:
Heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNP A2/B1) has been identified as a nuclear DNA sensor. Upon viral infection, hnRNP A2/B1 recognizes pathogen-derived DNA as a homodimer, which is a prerequisite for its translocation to the cytoplasm to activate the interferon response. However, the DNA binding mechanism inducing hnRNP A2/B1 homodimerization is unknown. Here, we show the crystal structure of the RNA recognition motif (RRM) of hnRNP A2/B1 in complex with a U-shaped ssDNA, which mediates the formation of a newly observed protein dimer. Our biochemical assays and mutagenesis studies confirm that the hnRNP A2/B1 homodimer forms in solution by binding to pre-generated ssDNA or dsDNA with a U-shaped bulge. These results depict a potential functional state of hnRNP A2/B1 in antiviral immunity and other cellular processes.
- The State Key Laboratory of Pharmaceutical Biotechnology, School of Life Sciences, Institute of Viruses and Infectious Diseases, Chemistry and Biomedicine Innovation Center (ChemBIC), Institute of Artificial Intelligence Biomedicine, Nanjing University, Nanjing, People's Republic of China.
Organizational Affiliation: